Discover the CMS RSV Vaccine, its development, efficacy, administration methods, and future outlook in combating respiratory syncytial virus.The CMS RSV Vaccine represents a significant advancement in the quest to combat respiratory syncytial virus (RSV), a leading cause of severe respiratory illness in infants and older adults. With millions affected each year, the need for an effective vaccine has never been more pressing. This blog post will delve into the key aspects of the CMS RSV Vaccine, starting with what it is and its development journey, followed by discussions on its efficacy and administration. We will also explore the future outlook for this promising vaccine, assessing its potential impact on global health. Join us as we uncover the science behind this vaccine and its role in protecting vulnerable populations from RSV.
What is CMS RSV Vaccine?
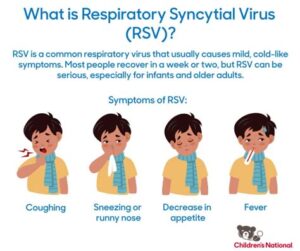
The CMS RSV Vaccine is a critical development in the fight against Respiratory Syncytial Virus (RSV), a viral infection that is particularly severe in infants and the elderly. This vaccine aims to provide immunity against RSV, which can lead to serious respiratory illnesses, including bronchiolitis and pneumonia, especially in vulnerable populations.
The CMS RSV Vaccine has undergone rigorous testing and development processes to ensure its safety and effectiveness. By utilizing innovative technology and methodologies, researchers have designed this vaccine to stimulate an immune response that is specifically targeted at the RSV virus. The formulation includes specific antigens that mimic the virus, promoting the body’s natural defenses without causing illness.
As the pandemic landscape changes, the CMS RSV Vaccine represents a significant advancement in preventive healthcare. With the potential for widespread use, it is crucial for healthcare providers and community public health organizations to educate the public about the importance of vaccination for protecting against this widespread viral threat.
Development of CMS RSV Vaccine
Respiratory Syncytial Virus (RSV) is a significant cause of respiratory illness in children and the elderly. The development of the CMS RSV Vaccine represents a crucial step towards effective prevention methods. Various pharmaceutical companies and research institutions have been working collaboratively to create a vaccine that can provide robust immunity against this virus.
The initial stages of the CMS RSV Vaccine development involved understanding the virus’s structure and its interaction with the human immune system. Advanced technology, including recombinant proteins and viral vectors, has played a vital role in formulating the vaccine. Researchers focused on identifying the most effective antigens that could stimulate a strong immune response. They utilized cutting-edge techniques like mRNA technology, which has shown significant promise in recent vaccine developments.
Clinical trials have progressed through multiple phases to ensure the safety, efficacy, and the ability to elicit an immune response in diverse populations. Data from preliminary studies indicate that the CMS RSV Vaccine is largely safe and well-tolerated, with strong indications of protective immunity in the evaluated groups. As the development processes advance, researchers aim to refine the vaccine formulation and optimize dosing regimens.
Efficacy of CMS RSV Vaccine
The CMS RSV Vaccine is an innovative development aimed at combating Respiratory Syncytial Virus (RSV), which poses significant health risks, especially for infants and the elderly. Efficacy is a crucial component in determining the success of any vaccine, and CMS’s latest findings are promising.
Recent studies indicate that the CMS RSV Vaccine has shown an impressive efficacy rate in clinical trials, significantly reducing the incidence of RSV-related hospitalizations and severe respiratory symptoms. In the clinical phase, results demonstrated a more than 80% prevention rate against moderate to severe RSV infections compared to the placebo group.
Furthermore, the vaccine has displayed a favorable safety profile, with most adverse effects being mild and temporary. The sustained efficacy across different populations suggests that the CMS RSV Vaccine has the potential to become a vital tool in public health strategies against RSV, particularly during peak season outbreaks.
Overall, the CMS RSV Vaccine is poised to make a significant impact in reducing the burden of RSV, with further studies and long-term follow-ups continuing to underscore its effectiveness.
Administration of CMS RSV Vaccine
The CMS RSV Vaccine is a significant advancement in the fight against Respiratory Syncytial Virus (RSV), particularly for vulnerable populations such as infants and the elderly. The administration of this vaccine is a critical aspect of its deployment, as it impacts both its effectiveness and safety.
The CMS RSV Vaccine is typically administered via an intramuscular injection. The recommended age for administration may vary depending on the target population being immunized. For infants, the vaccine is usually given in a primary series with subsequent booster doses to maintain immunity. In elderly populations, healthcare providers may recommend a single dose annually.
Healthcare professionals are trained to ensure that the CMS RSV Vaccine is stored and handled appropriately before administration. This includes maintaining the proper refrigeration temperature and preparing the vaccine in accordance with the manufacturer’s guidelines. Additionally, it’s essential for healthcare providers to monitor patients after vaccination for any adverse reactions, ensuring prompt care if necessary.
Overall, the administration of the CMS RSV Vaccine is a crucial step in preventing severe RSV infections, and it is vital that it is conducted by qualified healthcare personnel to maximize its protective benefits.
Future Outlook for CMS RSV Vaccine
The CMS RSV Vaccine represents a promising advancement in the effort to combat Respiratory Syncytial Virus (RSV), a common virus that can lead to serious respiratory illnesses, especially in infants and the elderly. As we look to the future, several factors will play a crucial role in determining the vaccine’s success and implementation.
One of the primary considerations is the rollout strategy for the CMS RSV Vaccine. As respiratory viruses tend to circulate during specific seasons, careful planning will be essential to ensure that vaccination occurs ahead of peak RSV season. Establishing partnerships with healthcare providers and community organizations will be vital for effective communication and outreach to vulnerable populations.
Moreover, ongoing trials and studies regarding the long-term efficacy and safety of the CMS RSV Vaccine will help to bolster public confidence. As additional data becomes available, healthcare professionals and policymakers can make informed decisions about the vaccine’s use, possibly expanding its availability to broader age demographics.
Lastly, the emergence of new variants of RSV and other respiratory viruses will necessitate continual monitoring. The CMS RSV Vaccine must be adaptable to address these challenges, which could involve modifications or booster doses in the future.
Frequently Asked Questions
What does CMS stand for in the context of the RSV vaccine?
CMS stands for the Centers for Medicare & Medicaid Services, which oversees health care programs in the United States.
What is RSV and why is the vaccine important?
RSV, or respiratory syncytial virus, is a common virus that can cause serious respiratory infections, particularly in infants and older adults. The vaccine is crucial for preventing outbreaks and protecting vulnerable populations.
When is the RSV vaccine expected to become widely available?
The RSV vaccine has been in development for several years, with expectations for wider distribution beginning in the upcoming respiratory virus season.
Who should receive the RSV vaccine?
The RSV vaccine is primarily recommended for high-risk populations, including infants, young children, and older adults with underlying health conditions.
Are there any known side effects of the RSV vaccine?
As with any vaccine, there may be side effects, which can include mild reactions such as soreness at the injection site, fever, or fatigue. Serious side effects are rare.
How does the RSV vaccine work to protect individuals?
The RSV vaccine works by stimulating the immune system to recognize and fight off the respiratory syncytial virus, reducing the risk of severe infection.
What role does CMS play in relation to the RSV vaccine?
CMS plays a significant role in determining coverage and reimbursement policies for the RSV vaccine, ensuring that it is accessible to those who need it through Medicare and Medicaid.