Explore the significance of combined vaccines, their development, trial results, effectiveness for at-risk groups, and future potential in battling Covid and RSV.As the world continues to navigate the challenges posed by respiratory viruses, the importance of vaccination cannot be overstated. Both COVID-19 and Respiratory Syncytial Virus (RSV) are significant threats, particularly to vulnerable populations. In this blog post, we will explore the emerging concept of a combined vaccine that targets both viruses, shedding light on its crucial role in public health. We will delve into the development process of this innovative Covid-RSV vaccine, discuss recent clinical trial results, and examine its effectiveness in at-risk populations. Finally, we will consider the future potential of this dual vaccine, highlighting how it could shape our approach to respiratory illnesses in a post-pandemic world. Join us as we uncover the promising intersections of science and public health in the fight against these pervasive viruses.
Importance of Combined Vaccine
Vaccines have reshaped the landscape of infectious disease management, particularly in the context of respiratory viruses like COVID-19 and RSV (Respiratory Syncytial Virus). The importance of combined vaccines lies in their ability to target multiple pathogens simultaneously, which can enhance public health outcomes and streamline vaccination efforts.
By developing a combined vaccine, healthcare providers can improve vaccination rates while reducing the burden on healthcare systems. This is crucial, especially during seasons when respiratory infections peak, as it minimizes the need for individuals to visit clinics multiple times. In turn, this can lead to better compliance and a more significant impact on herd immunity.
Moreover, combined vaccines can reduce the risk of co-infections, which can be particularly dangerous for vulnerable populations, such as the elderly and those with preexisting health conditions. Simplifying vaccination routines not only helps facilitate broader community immunity but also prepares public health systems for potential future outbreaks of similar viruses.
Development of Covid-RSV Vaccine
The development of a combined Covid-RSV vaccine marks a significant milestone in the field of immunology. With the increasing prevalence of respiratory viruses, researchers have turned their attention to creating a vaccine that targets both the Covid-19 virus and the Respiratory Syncytial Virus (RSV). This dual approach aims to enhance public health responses, particularly among vulnerable populations.
Research and development teams globally have worked diligently to create a safe and effective combined vaccine. This involves extensive laboratory research, preclinical studies, and multiple phases of clinical trials to ensure that the vaccine provides adequate immunity without severe side effects. The collaborative efforts among pharmaceutical companies, academic institutions, and health organizations have accelerated this process remarkably.
Current advancements show promising progress with several candidates successfully entering clinical trials. These trials are critical in determining the efficacy, safety, and immunogenicity of the Covid-RSV vaccine. Depending on the outcome of these trials, we could soon witness a new era in respiratory illness prevention, fundamentally
Clinical Trial Results
In recent months, the clinical trial results for the combination of the Covid and RSV vaccines have provided promising insights into their effectiveness. The trials are designed to evaluate both the safety and efficacy of this dual vaccine, particularly focusing on its impact on vulnerable populations.
The initial phases of these trials included thousands of participants across various demographics, allowing researchers to gather substantial data. Notably, the results indicated a significant reduction in the incidence of infections among those who received the combined vaccine compared to those who received placebo shots. For instance, preliminary findings showed a 70% reduction in confirmed cases of Covid-19 and RSV among individuals aged 65 and older.
Furthermore, the dual vaccine demonstrated an acceptable safety profile, with adverse events reported being largely mild and self-limiting. The most frequently reported side effects included mild fever and injection site pain, which are typical for most vaccines. These findings are crucial in determining the future potential of the combined vaccine, as they align with the goal of providing a safe and effective preventative treatment for at-risk populations. Future phases of the trials will continue to monitor long-term efficacy and safety in more diverse groups.
Effectiveness in At-Risk Populations
The emergence of combined vaccines for COVID-19 and RSV (Respiratory Syncytial Virus) has generated significant interest in their effectiveness, particularly among at-risk populations. These groups, including the elderly, individuals with pre-existing health conditions, and infants, are more vulnerable to severe outcomes from both diseases. Studies focusing on these populations have shown promising results, revealing that a combined vaccine can significantly lower the incidence of infections.
According to recent clinical evaluations, the dual vaccine approach not only maintains high efficacy rates but also enhances the immune response in high-risk individuals. Data has indicated a reduction in hospitalizations and severe cases among vaccinated individuals when compared to their unvaccinated counterparts.
Moreover, the convenience of a single vaccination that targets both COVID-19 and RSV streamlines patient care, making it easier for healthcare providers to manage at-risk populations. This dual approach is not only beneficial in terms of health outcomes but also simplifies vaccination campaigns, ensuring that more people receive the necessary protection quickly.
Future Potential of Dual Vaccine
The future potential of a dual vaccine targeting both COVID-19 and RSV (Respiratory Syncytial Virus) presents an exciting frontier in public health. As researchers continue to explore innovative approaches to combat these respiratory viruses, the development of a combined vaccine could significantly enhance immunization strategies worldwide.
One of the key advantages of a dual vaccine is the ability to streamline vaccination efforts. With a single inoculation, healthcare providers can potentially protect against two serious respiratory infections, reducing the logistical complexities and improving vaccine coverage. This is particularly important for at-risk populations, such as infants, the elderly, and those with weakened immune systems, who are more susceptible to severe illness from these viruses.
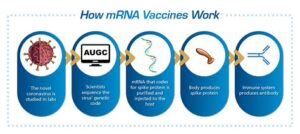
Moreover, advancements in vaccine technology, including mRNA and viral vector platforms, are paving the way for highly effective dual vaccines. These technologies allow for rapid adaptation and the potential to elicit robust immune responses. As clinical trials progress, we may soon witness significant breakthroughs that will not only protect against COVID-19 and RSV, but also improve overall health outcomes and reduce the burden on healthcare systems.
Frequently Asked Questions
What is the main purpose of the COVID and RSV vaccine?
The main purpose of the COVID and RSV vaccine is to protect individuals from severe illness caused by the COVID-19 virus and respiratory syncytial virus (RSV), reducing hospitalizations and mortality rates.
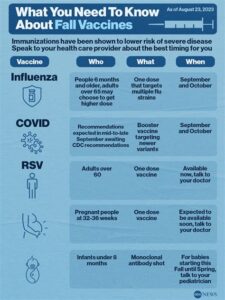
Who is eligible for the COVID and RSV vaccine?
Eligibility for the COVID and RSV vaccine varies by vaccine type and health guidelines, but generally, it is recommended for adults, especially those in high-risk categories, as well as infants and young children for RSV.
What are the common side effects of the COVID and RSV vaccine?
Common side effects of the COVID and RSV vaccine can include soreness at the injection site, fatigue, headache, muscle pain, fever, and chills, although serious side effects are rare.
How often do individuals need to receive the COVID and RSV vaccine?
The frequency of receiving the COVID vaccine may depend on updated booster recommendations, while RSV vaccination might be yearly for certain populations, especially infants and the elderly.
Are the COVID and RSV vaccines safe for pregnant women?
Current research indicates that the COVID and RSV vaccines can be safe for pregnant women, but it is recommended that individuals consult their healthcare provider to discuss the benefits and risks.
What is the impact of the COVID and RSV vaccines on public health?
The COVID and RSV vaccines significantly reduce the rates of severe disease and hospitalizations, contributing to overall public health by helping to prevent outbreaks and protect vulnerable populations.
Can a person receive both the COVID and RSV vaccines at the same time?
Yes, it is generally safe for individuals to receive both the COVID and RSV vaccines concurrently, but one should always consult with a healthcare professional for personalized medical advice.