Discover the CPT RSV Vaccine’s definition, development, effectiveness, side effects, and future prospects in combating respiratoRespiratory Syncytial Virus (RSV) is a leading cause of respiratory illness in young children and vulnerable populations, making effective preventive measures crucial. The CPT RSV vaccine represents a promising advancement in combating this widespread virus. In this blog post, we will explore the CPT RSV vaccine in depth, beginning with an overview of what it entails and its development journey. We will also examine its effectiveness and potential side effects to provide a comprehensive understanding. Furthermore, we will discuss the future directions of the CPT RSV vaccine and the impact it may have in public health initiatives. Join us as we delve into this groundbreaking vaccine that could change the landscape of RSV prevention and protection for generations to come.
What is the CPT RSV Vaccine?
The CPT RSV Vaccine is a cutting-edge immunization designed to protect against Respiratory Syncytial Virus (RSV), a major cause of respiratory illness in infants and young children. RSV can lead to severe respiratory infections, particularly in vulnerable populations such as premature infants, children with congenital heart disease, and those with weakened immune systems.
This vaccine uses a Conjugate Protein Technology (CPT) approach, which enhances the immune response by incorporating specific RSV proteins that stimulate the body’s natural defenses without causing the disease itself. As a result, the CPT RSV Vaccine aims to elicit a strong and lasting immune response to protect against future infections.
The importance of the CPT RSV Vaccine cannot be overstated, as RSV leads to thousands of hospitalizations each year in children under the age of five. By providing effective immunity, this vaccine could significantly reduce the incidence of these hospitalizations and associated healthcare costs.
Development of the CPT RSV Vaccine
Respiratory Syncytial Virus (RSV) is a significant cause of respiratory illness in infants and children. The development of the CPT RSV Vaccine has been a critical advancement in the fight against this virus. Researchers recognized the urgent need for a vaccine due to the high hospitalization rates associated with RSV infections.
The journey towards creating the CPT RSV Vaccine began with a deep understanding of the virus’s biology. Scientists focused on identifying the most effective ways to stimulate the immune system to protect against RSV. Initial studies explored various vaccine platforms, leading to the selection of a recombinant protein-based approach, which shows promise in eliciting robust immune responses.
Collaborations between public health organizations, pharmaceutical companies, and research institutions played a pivotal role in accelerating the vaccine’s development. Clinical trials provided critical data on the vaccine’s safety and efficacy. As research progressed, the need for a seasonal vaccination strategy became evident, allowing for better immune protection during peak RSV seasons.
| Development Phase | Key Activities | Goals |
|---|---|---|
| Preclinical Trials | Laboratory and animal studies | Assess safety and immunogenicity |
| Phase I Trials | Testing on healthy adults | Evaluate safety and dosage |
| Phase II Trials | Testing on infants and children | Assess efficacy and side effects |
| Phase III Trials | Large-scale testing | Confirm efficacy in a broader population |
The CPT RSV Vaccine represents the culmination of years of research and development, with the potential to change the landscape of RSV prevention. As we look to the future, continual advancements and studies will further enhance our understanding of vaccine effectiveness, ensuring
Effectiveness of the CPT RSV Vaccine
The effectiveness of the CPT RSV Vaccine has become a significant topic of discussion among healthcare professionals and researchers. This vaccine is designed to combat Respiratory Syncytial Virus (RSV), which is notorious for affecting infants and young children, leading to severe respiratory issues. The clinical trials surrounding the vaccine have shown promising results.
Studies indicate that the CPT RSV Vaccine has an efficacy rate of around 70-80% in preventing serious RSV infections in infants. This is especially critical since RSV is one of the leading causes of hospitalization in children under the age of two. Moreover, the vaccine has been found to provide a robust immune response, equipping the body to fight off RSV more effectively compared to unvaccinated individuals.
In addition to its effectiveness in preventing infections, the CPT RSV Vaccine has also demonstrated the ability to reduce the severity of RSV symptoms in vaccinated children who do contract the virus. This dual benefit is vital in managing healthcare costs and improving overall patient outcomes, making the vaccine an essential tool in the fight against RSV.
Side Effects of the CPT RSV Vaccine
The CPT RSV Vaccine has emerged as a promising treatment option for respiratory syncytial virus (RSV), especially in vulnerable populations such as infants and the elderly. However, like any medical intervention, it is essential to be aware of the potential side effects that may arise following vaccination.
| Side Effect | Description |
|---|---|
| Injection Site Reactions | Pain, redness, or swelling at the site of injection |
| Fever | A mild fever may occur as the immune system responds to the vaccine |
| Fatigue | Some individuals might experience tiredness or lethargy |
| Headache | Headaches can occur as a common reaction to vaccination |
While these side effects are generally mild and resolve within a few days, it is crucial to monitor for any adverse reactions. In rare cases, individuals may experience more severe side effects, including allergic reactions such as hives or difficulty breathing. In such instances, immediate medical attention should be sought.
It is worth noting that the benefits of the CPT RSV Vaccine, particularly its potential to prevent severe RSV infections, often outweigh the risks associated with these potential side effects. Before receiving the vaccine, individuals are encouraged to discuss any concerns with their healthcare provider to ensure a well-informed decision.
Future of the CPT RSV Vaccine
The CPT RSV Vaccine holds great promise for the future of respiratory syncytial virus (RSV) prevention, particularly for vulnerable populations such as infants and the elderly. As RSV continues to pose significant health risks worldwide, ongoing research and advancements in vaccine technology aim to enhance the vaccine’s efficacy and accessibility.
In the coming years, we can anticipate several exciting developments in the field of the CPT RSV Vaccine. Researchers are actively exploring innovative formulations and delivery methods to improve immunogenicity and safety profiles. For instance, newer adjuvants and combination vaccines could potentially boost the body’s immune response, offering broader and longer-lasting protection against RSV.
Additionally, global collaboration and funding initiatives are likely to increase, facilitating clinical trials in diverse populations and ensuring that the CPT RSV Vaccine is tailored to meet different health care needs. This collaboration will be crucial in making the vaccine accessible to low and middle-income countries, where RSV burden is particularly high.
In summary, the future of the CPT RSV Vaccine is bright, marked by innovative research and dedication towards broadening its reach and effectiveness. With continued efforts, this vaccine could significantly reduce the incidence of RSV-related complications and hospitalizations in vulnerable populations around the world.
Frequently Asked Questions
What is the CPT RSV vaccine?
The CPT RSV vaccine is a vaccine specifically designed to protect against respiratory syncytial virus (RSV), which is a common viral infection that can cause severe respiratory illness in young children and vulnerable populations.
Who should receive the CPT RSV vaccine?
The CPT RSV vaccine is typically recommended for infants and young children, particularly those who are at higher risk for severe RSV infection, such as premature infants or those with underlying health conditions.
How does the CPT RSV vaccine work?
The CPT RSV vaccine works by stimulating the immune system to create a response against the RSV virus, thereby building immunity and reducing the likelihood of severe illness if exposed to the virus.
When is the best time to administer the CPT RSV vaccine?
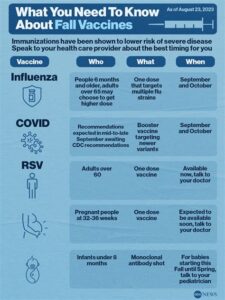
The best time to administer the CPT RSV vaccine is usually during the RSV season, which varies by region, but it generally occurs in the fall and winter months.
Are there any side effects associated with the CPT RSV vaccine?
Like all vaccines, the CPT RSV vaccine may cause mild side effects such as soreness at the injection site, low-grade fever, or irritability, but serious side effects are rare.
How effective is the CPT RSV vaccine?
Clinical studies have shown that the CPT RSV vaccine is effective in reducing the incidence of RSV-related hospitalizations and severe respiratory illness among vaccinated children.
Is the CPT RSV vaccine part of the standard vaccination schedule?
The CPT RSV vaccine may not be part of the standard vaccination schedule but is recommended as an additional preventive measure for at-risk populations during RSV season.