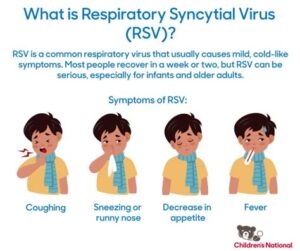
Discover key insights about the RSV vaccine, including its components, safety, and misconceptions to help you make informed health decisions.In recent years, respiratory syncytial virus (RSV) has garnered significant attention, particularly in the context of vaccine development aimed at preventing this common yet potentially severe respiratory infection. As public interest in the RSV vaccine grows, many people are left with pressing questions about its formulation and safety. A key point of curiosity is whether the RSV vaccine contains a live virus, a concern that often stems from misunderstandings surrounding vaccination practices. In this blog post, we will delve into the intricacies of the RSV vaccine, exploring its components, assessing safety concerns, and clarifying common misconceptions. By providing accurate information, we aim to empower readers to make informed decisions about their health and the health of their loved ones in light of the ongoing research and developments in RSV immunization.
Understanding the RSV Vaccine
The Respiratory Syncytial Virus (RSV) vaccine has been a significant focus in the world of healthcare due to the virus’s impact on infants and the elderly. Unlike some vaccines that utilize live pathogens, the RSV vaccine does not contain a live virus. Instead, it incorporates various components that help stimulate the immune system to recognize and combat RSV without the associated risks of an active infection.
This vaccine primarily uses a method known as subunit technology, which involves isolating specific proteins from the RSV that are crucial for inducing an immune response. These proteins act as antigens, triggering the body’s immune system to prepare for future encounters with the virus, thereby providing protective benefits.
Furthermore, ongoing research ensures that the vaccine’s formulation is both safe and effective. Regulatory bodies conduct rigorous testing to evaluate the safety of the RSV vaccine, ensuring that it meets all health standards before being made available to the public. This meticulous process is essential in building trust and addressing any safety concerns that may arise in discussions about vaccination.
Exploring the Vaccine Components
When it comes to understanding the RSV vaccine, it’s essential to delve into the components that make up the formulation. The *Respiratory Syncytial Virus* (RSV) vaccine is designed to stimulate the body’s immune response without causing disease.
| Component | Function |
|---|---|
| Antigen | Stimulates immune response |
| Adjuvant | Enhances the effectiveness of the vaccine |
| Stabilizers | Maintain vaccine potency |
| Preservatives | Prevents contamination |
The antigen is a key player in the RSV vaccine, as it is the part that prompts the immune system to recognize and fight off the actual RSV virus. This response is crucial in developing immunity, leading to fewer hospitalizations and complications associated with RSV infections.
Additionally, adjuvants are added to many vaccines, including the RSV vaccine, to boost the body’s response. They play a vital role in ensuring that the immune system takes note of the antigen and prepares to combat any future exposure to the virus.
Finally, vaccines may include stabilizers and preservatives to ensure the vaccine’s safety and efficacy throughout its shelf life, ensuring that recipients receive a potent dose when needed. Understanding these components is fundamental in demystifying the RSV vaccine and recognizing its significance in public health.
Investigating the Presence of Live Virus
One of the most common concerns surrounding vaccines is whether they contain a live virus that could potentially cause infection. In the case of the RSV vaccine, this inquiry is particularly pertinent. Understanding the formulation of this vaccine can help alleviate fears and misconceptions.
The RSV vaccine currently in development and evaluation does not contain a live virus capable of causing disease. Instead, the vaccine employs modern vaccine technologies, which may include inactivated virus, subunit components, or mRNA technology, designed to elicit an immune response without the risks associated with live pathogens.
| Type of Vaccine | Contains Live Virus? |
|---|---|
| Live Attenuated Vaccines | Yes |
| Inactivated Vaccines | No |
| Subunit Vaccines | No |
| mRNA Vaccines | No |
It is essential to emphasize that the RSV vaccine does not involve any live viruses that could make someone sick, thereby ensuring a safer option for individuals,
Evaluating Vaccine Safety Concerns
When it comes to the RSV vaccine, safety is a critical concern for both healthcare providers and patients. Vaccines are subject to rigorous testing before they are approved for public use, and the RSV vaccine is no exception. This vaccine has undergone extensive clinical trials to evaluate its efficacy and safety profile.
One common safety concern is the possibility of adverse reactions. While some individuals may experience mild side effects such as soreness at the injection site or mild fever, serious reactions are extremely rare. The regulatory bodies that oversee vaccination programs, such as the FDA and CDC, continuously monitor data from vaccine recipients to ensure ongoing safety.
Furthermore, understanding the composition of the RSV vaccine can alleviate concerns. Unlike some vaccines that contain live viruses, the RSV vaccine is formulated using inactivated or non-replicating components. This means that it cannot cause the disease it is designed to protect against,
Clarifying Misconceptions about the RSV Vaccine
Many people have misconceptions about the Respiratory Syncytial Virus (RSV) vaccine, leading to confusion and hesitation towards vaccination. One common myth is the idea that the RSV vaccine contains a live virus. Understanding the formulation of this vaccine is essential to clear up these misunderstandings.
In reality, the RSV vaccine is designed using an inactivated or attenuated virus, meaning it does not contain any live viral particles that can replicate or cause the disease. The aim is to stimulate the immune system to develop a robust response without posing any risk of causing RSV itself. This distinction is vital for anyone considering receiving the vaccine, as it directly influences their perception of safety.
Moreover, multiple studies have reinforced the notion that vaccines containing live virus can lead to complications in certain populations. Conversely, the RSV vaccine’s components have undergone rigorous testing to ensure they are both effective and safe for public use. It’s crucial to engage with credible sources an
Frequently Asked Questions
What is the RSV vaccine?
The RSV vaccine is designed to protect against respiratory syncytial virus (RSV), which is a common viral infection that can lead to severe respiratory illness, especially in infants and older adults.
Does the RSV vaccine contain live virus?
No, the current RSV vaccines do not contain live virus. They are made using either inactivated viruses or specific proteins from the virus to stimulate an immune response.
Who should get the RSV vaccine?
The RSV vaccine is primarily recommended for high-risk populations such as infants born prematurely, those with underlying health conditions, and older adults with certain health risks.
What are the benefits of the RSV vaccine?
The RSV vaccine can significantly reduce the risk of RSV infections, hospitalizations, and associated complications, ultimately leading to improved health outcomes for vulnerable populations.
Are there any side effects associated with the RSV vaccine?
Like any vaccine, the RSV vaccine may have side effects, which can include mild symptoms such as soreness at the injection site, fever, or fatigue. Serious side effects are rare.
When is the best time to get the RSV vaccine?
The ideal timing for receiving the RSV vaccine can vary based on individual risk factors, but it is typically administered during the fall and winter months when RSV circulation is highest.
Will the RSV vaccine protect against all strains of RSV?
While the RSV vaccine is designed to provide broad protection against the most common strains, it may not cover every strain of RSV. Ongoing research aims to improve the efficacy of the vaccine.