Learn about the RSV vaccine, its mechanism, infection process, clinical trials, and effectiveness, along with potential side effects for informed health decisions.As the world increasingly confronts respiratory viruses, the respiratory syncytial virus (RSV) has emerged as a significant concern, particularly for infants and vulnerable populations. Vaccination represents a powerful strategy to combat this virus and reduce its impact. In this blog post, we delve into the intricacies of the RSV vaccine—what it is, how it works within the body, and the mechanisms that underpin its action. Moreover, we’ll explore the latest clinical trials and research findings to better understand its effectiveness and potential side effects. Join us as we unravel the science behind this vital vaccine and its role in protecting our communities from the threats posed by RSV.
What is the RSV vaccine?
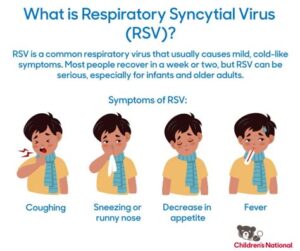
Respiratory Syncytial Virus (RSV) is a common viral infection that primarily affects infants and young children, but it can also impact adults, especially the elderly. The RSV vaccine is developed to protect against this potentially serious respiratory virus, which can lead to severe respiratory health issues, including pneumonia and bronchiolitis.
The RSV vaccine works by stimulating the immune system to produce specific antibodies against the virus. These antibodies help the body recognize and fight the virus more effectively if exposed in the future. Vaccination is particularly crucial for high-risk groups, such as premature infants and those with underlying health conditions, as they are more susceptible to severe RSV infections.
While various formulations of the RSV vaccine are being studied, they generally aim to provide immunity without causing the disease. The development process includes extensive clinical trials to ensure both the efficacy and safety of the vaccine. As research advances, the goal remains to provide a reliable means of preventing RSV infections and reducing hospitalizations related to the virus.
How does RSV infect the body?
Respiratory Syncytial Virus, commonly known as RSV, is a major cause of respiratory infections, particularly in infants and young children. The virus primarily spreads through respiratory droplets when an infected person coughs, sneezes, or even talks. Once these droplets are inhaled, the virus targets the epithelial cells in the upper respiratory tract.
After entering the body, RSV begins its infection process by first attaching to the surface of the epithelial cells. This attachment is facilitated by proteins on the virus’s surface, which bind to specific receptors on the host cells. Following this attachment, the virus enters the cells and begins to replicate, resulting in cell damage and inflammation. The patient often experiences symptoms resembling common cold or flu, including wheezing and difficulty breathing.
As the infection progresses, the immune system responds to the presence of RSV. However, in many cases, particularly with young children, the immune response can lead to significant inflammation of the airways, which further complicates breathing and can lead to bronchiolitis or pneumonia. Understanding how RSV infects the body is crucial for developing effective vaccines and treatments against this common yet potentially serious virus.
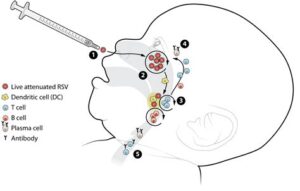
Mechanism of action of RSV vaccine
The Respiratory Syncytial Virus (RSV) is a significant cause of respiratory infections, particularly in infants, older adults, and immunocompromised individuals. Understanding the mechanism of action of the RSV vaccine is crucial in combating these infections. The vaccine aims to stimulate the immune system to recognize and effectively respond to RSV upon exposure.
The RSV vaccine works primarily by introducing a harmless part of the virus, often in the form of an inactivated virus or specific proteins from the virus, into the body. This exposure prompts the immune system to produce antibodies, which are specialized proteins that can identify and neutralize the virus. These antibodies enable the body to remember and recognize RSV in future exposures, thus providing immunity.
- Antigen Recognition: The immune system identifies the viral components provided by the vaccine as foreign invaders.
- Antibody Production: B cells, a type of white blood cell, are activated to produce antibodies specific to RSV.
- Memory Cell Formation: Some B cells become memory cells, allowing for rapid and robust responses to subsequent RSV infections.
This process is fundamental in protecting vulnerable populations from the severe effects of RSV, as it ensures that their immune systems are primed to act swiftly against the virus.
Clinical trials and research on RSV vaccine
Respiratory Syncytial Virus (RSV) is a significant cause of respiratory infections, especially in infants and older adults. With the increasing burden of RSV-related hospitalizations, there has been a concerted effort in the medical community to develop effective vaccines. Clinical trials are a crucial component of this process and provide valuable insights into the vaccine’s safety and effectiveness.
Throughout various phases of clinical trials, researchers have focused on evaluating the safety profiles of RSV vaccines, their ability to induce an immune response, and their effectiveness in preventing RSV infections. For instance, the most recent trials have tested modified live-attenuated vaccines, recombinant protein vaccines, and vector-based vaccines to find the optimal approach.
| Phase | Description | Key Findings |
|---|---|---|
| Phase 1 | Initial safety assessments in healthy adult volunteers. | Demonstrated good safety profiles with minimal side effects. |
| Phase 2 | Larger cohort including infants and older adults to evaluate immune responses. | Induced strong antibody responses, especially in older adult populations. |
| Phase 3 | Large-scale trials to determine effectiveness. | Reported significant reductions in RSV infection rates among vaccinated individuals. |
Continued research is essential as it helps refine vaccine formulations and dosing schedules. The data collected from these clinical trials guide regulatory approvals and influence public health recommendations. As the results from these trials become available, they will ultimately shape the future of RSV prevention and help reduce the global burden of this virus.
Effectiveness and side effects of RSV vaccine
The RSV vaccine has emerged as a crucial tool in the fight against respiratory syncytial virus (RSV), particularly in vulnerable populations such as infants and the elderly. Understanding the effectiveness of this vaccine is essential for public health insights. Clinical studies have shown that the vaccine significantly reduces the incidence of RSV-related hospitalizations and severe respiratory illnesses.
In terms of effectiveness, recent clinical trials indicate that the RSV vaccine can reduce the severity of the illness by up to 70-80%, depending on the population and specific strains of the virus. This level of protection is crucial for high-risk groups who are susceptible to severe complications from RSV. Individuals vaccinated typically experience milder symptoms if they do contract the virus, further emphasizing the importance of vaccination in controlling outbreaks.
However, like any medical intervention, the RSV vaccine may come with some side effects. Common side effects noted in clinical trials include mild symptoms such as fever, fatigue, and local pain at the injection site. These symptoms are generally short-lived and resolve on their own. In rare cases, some individuals might experience more severe allergic reactions, though this is not prevalent among the broader population. Monitoring continues to ensure the ongoing safety and efficacy of the vaccine.
Frequently Asked Questions
What is the RSV vaccine designed to prevent?
The RSV vaccine is designed to prevent respiratory syncytial virus (RSV) infections, which can lead to severe respiratory illnesses, especially in infants and older adults.
How does the RSV vaccine work in the body?
The RSV vaccine works by stimulating the immune system to recognize and fight off the RSV virus, producing antibodies that protect against future infections.
Who should receive the RSV vaccine?
The RSV vaccine is recommended primarily for infants, young children, and high-risk adults, such as those with chronic respiratory conditions or weakened immune systems.
Are there any side effects associated with the RSV vaccine?
Like any vaccine, the RSV vaccine can have side effects, which may include mild symptoms such as fever, soreness at the injection site, and fatigue.
When is the best time to administer the RSV vaccine?
The optimal timing for administering the RSV vaccine is typically before the peak RSV season, which can vary by region but usually occurs in fall and winter.
Is the RSV vaccine safe for pregnant women?
Current guidelines suggest that pregnant women should consult their healthcare provider, as the safety of the RSV vaccine during pregnancy is still under research.
How effective is the RSV vaccine in preventing infections?
The effectiveness of the RSV vaccine can vary, but studies indicate it significantly reduces the risk of severe RSV illness in vaccinated populations.