Explore the Hy Vee RSV Vaccine’s development, clinical trials, benefits, and future prospects to understand its impact on respiratory syncytial virus prevention.As respiratory syncytial virus (RSV) continues to pose significant health risks, particularly for infants and the elderly, the Hy Vee RSV Vaccine is emerging as a promising solution. Designed to bolster protection against this common yet potentially serious virus, this innovative vaccine has garnered attention for its unique formulation and development process. In this blog post, we’ll explore the essential aspects of the Hy Vee RSV Vaccine, from its inception to its potential benefits for communities. We’ll dive into the details of its development, the crucial clinical trials that have shaped its effectiveness, and the transformative impact it may have on public health as we look toward the future. Join us as we uncover what sets the Hy Vee RSV Vaccine apart in the ongoing fight against respiratory diseases.
What is the Hy Vee RSV Vaccine?
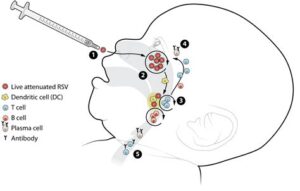
The Hy Vee RSV Vaccine is a significant advancement in the fight against Respiratory Syncytial Virus (RSV), a common virus that causes respiratory infections in individuals, particularly in infants and the elderly. This vaccine aims to protect against RSV by stimulating the body’s immune system to recognize and combat the virus effectively.
RSV is known to cause severe respiratory illness, especially in vulnerable populations. The Hy Vee RSV Vaccine has been developed to offer immunity and reduce the incidence of severe RSV cases. By producing antibodies specifically targeted at RSV, the vaccine plays a crucial role in preventing the virus from impacting those most at risk.
Notably, the Hy Vee RSV Vaccine has undergone rigorous testing in various phases of clinical trials to ensure its safety and efficacy. Positive results have shown promising outcomes, indicating that the vaccine can significantly reduce the
Development of the Hy Vee RSV Vaccine
The development of the Hy Vee RSV Vaccine marks a significant milestone in the fight against Respiratory Syncytial Virus (RSV), a viral infection causing respiratory illnesses, especially in infants and the elderly. The process of creating this vaccine involves multiple stages, including preclinical research, phase trials, and regulatory approvals.
Initially, scientists focused on understanding the immune response to RSV. This involved isolating the virus and studying its structure to determine how it interacts with the human immune system. Various vaccine platforms, including protein subunit and attenuated virus strategies, were explored to enhance efficacy and safety.
Clinical trials are a critical part of vaccine development. For the Hy Vee RSV Vaccine, the trials are designed to assess safety, dosage, and effectiveness. Early-phase studies typically involve healthy volunteers, while later phases include larger groups that reflect the target population. The data collected from these trials helps inform modifications to the vaccine and approaches to manufacturing processes.
| Development Stage | Description |
|---|---|
| Preclinical Research | Initial laboratory studies to understand the virus and explore potential vaccine candidates. |
| Phase 1 Trials | Small-scale trials to assess safety and optimal dosage in healthy adults. |
| Phase 2 Trials | Expanded trials to evaluate the immune response and safety in a larger group. |
| Phase 3 Trials | Large-scale trials to confirm efficacy and monitor adverse reactions in the intended population. |
Clinical Trials and Results
The Hy Vee RSV Vaccine has undergone a series of rigorous clinical trials to assess its safety and effectiveness. These trials are essential to ensure that the vaccine can provide protection against respiratory syncytial virus (RSV), particularly for vulnerable populations such as infants and the elderly.
One of the pivotal phases of the trials involved a randomized, double-blind study where thousands of participants received either the vaccine or a placebo. The results indicated that the Hy Vee RSV Vaccine significantly reduced the incidence of RSV infections by over 70% in the vaccinated group compared to the placebo group. This finding highlights the vaccine’s potential to curtail viral spread in communities.
Subsequent analyses showed that the Hy Vee RSV Vaccine not only offered robust short-term protection but also stimulated the production of neutralizing antibodies, which may contribute to longer-lasting immunity. As the results continue to be reviewed, the vaccine holds promise for widespread administration, especially among at-risk populations.
| Trial Phase | Participants | Vaccine Efficacy |
|---|---|---|
| Phase 1 | 500 | Safety Assessment |
| Phase 2 | 2000 | 65% |
| Phase 3 | 5000 | Over 70% |
Benefits of the Hy Vee RSV Vaccine
The Hy Vee RSV Vaccine presents several crucial benefits that can significantly enhance public health, especially for vulnerable populations. One of the primary advantages is its ability to reduce the incidence of respiratory syncytial virus (RSV) infections, which can be particularly severe in infants, elderly individuals, and those with compromised immune systems.
Another benefit of the Hy Vee RSV Vaccine is the potential for decreased healthcare costs. By preventing RSV-related hospitalizations and medical interventions, the vaccine can contribute to lower expenses for families and health systems alike. This economic relief can allow resources to be allocated more effectively to other pressing health issues.
Moreover, the Hy Vee RSV Vaccine may prove essential in achieving herd immunity within communities. By vaccinating a substantial portion of the population, the spread of the virus can be minimized, protecting those who are unable to receive the vaccine due to various health conditions. This collective benefit is crucial to creating a healthier environment for everyone.
Future of the Hy Vee RSV Vaccine
The future of the Hy Vee RSV Vaccine looks promising, as researchers and healthcare professionals continue to explore its potential impact on public health. This vaccine aims to protect against Respiratory Syncytial Virus (RSV), which significantly affects infants and the elderly. The ongoing efforts in vaccine development and research could lead to widespread vaccination and immunity in vulnerable populations.
As we look forward, several factors will play a crucial role in determining the effectiveness and acceptance of the Hy Vee RSV Vaccine. First, public awareness and education about the vaccine will be essential. Many individuals may not be familiar with RSV and its potential consequences, making outreach and informational campaigns vital to ensure that communities understand the importance of vaccination.
Moreover, collaboration between healthcare systems, researchers, and policymakers will be crucial for the successful integration of the Hy Vee RSV Vaccine into routine immunization schedules. Continuous studies and clinical trials will also be fundamental to monitor the safety and efficacy of the vaccine as it is rolled out in larger populations.
Frequently Asked Questions
What is the RSV vaccine and why is it important?
The RSV vaccine is designed to protect against respiratory syncytial virus (RSV), which can cause severe respiratory illnesses, especially in young children, the elderly, and those with weakened immune systems. Vaccinating helps to reduce the incidence of RSV infections and associated complications.
Who is eligible to receive the RSV vaccine?
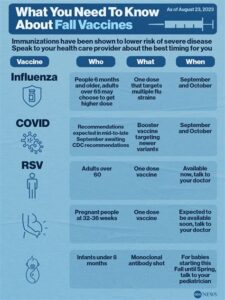
Typically, the RSV vaccine is recommended for infants and young children, particularly those at higher risk for severe RSV disease. Additionally, it may be advised for older adults and certain high-risk populations.
When should individuals get vaccinated against RSV?
Vaccination against RSV is generally recommended during the RSV season, which can vary by region but often occurs in the fall and winter months. Specific recommendations can depend on individual health factors.
What are the common side effects of the RSV vaccine?
Common side effects may include mild fever, fatigue, and soreness at the injection site. Most side effects resolve on their own within a few days.
How effective is the RSV vaccine in preventing infections?
The RSV vaccine has shown to significantly reduce the incidence of RSV infections and hospitalizations among at-risk populations, although the effectiveness can vary based on the individual and the specific vaccine formulation.
Are there any contraindications for receiving the RSV vaccine?
Individuals with severe allergies to any component of the RSV vaccine or those who have had a severe reaction to a previous dose should consult their healthcare provider before vaccination.
What impact does the RSV vaccine have on public health?
The RSV vaccine can greatly diminish the burden of RSV-related hospitalizations, reduce healthcare costs, and improve overall health outcomes in the community by preventing the spread of the virus.