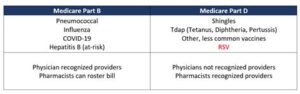
Explore the RSV vaccine, its development, effectiveness, side effects, and future prospects to understand its role in respiratory health.As the pursuit of effective immunizations continues, the respiratory syncytial virus (RSV) vaccine stands at the forefront of public health discussions. RSV is a leading cause of respiratory infections, particularly in infants and vulnerable populations, making the need for a reliable vaccine paramount. This blog post will explore the intricacies of the RSV vaccine, focusing on whether it is a live vaccine. We’ll delve into its development journey, assess its effectiveness, discuss potential side effects, and speculate on future prospects. Understanding the nuances of this vaccine is essential for parents and healthcare providers alike, as it could significantly impact the health and safety of our youngest and most susceptible individuals. Join us as we unravel the progress made in the quest for an RSV vaccine.
What is the RSV vaccine?
The RSV vaccine is designed to protect individuals from the respiratory syncytial virus (RSV), a common virus that can lead to serious respiratory infections, particularly in young children, the elderly, and those with weakened immune systems. RSV is responsible for many hospitalizations of infants and can cause bronchiolitis and pneumonia.
Currently, there are several types of RSV vaccines in development, primarily focusing on preventing the severe manifestations of the disease. The main goal of these vaccines is to stimulate the immune system to recognize and combat RSV effectively, reducing the incidence and severity of infections among at-risk populations.
Research on the RSV vaccine has made significant progress, including various approaches such as live attenuated vaccines and protein-based vaccines. The development team is focused on ensuring that the vaccines are both safe and effective for the target groups, particularly vulnerable populations like infants and the elderly.
Development of the live RSV vaccine
The development of the live RSV vaccine has been a long and challenging journey, marked by significant scientific achievements and numerous hurdles. Respiratory Syncytial Virus (RSV) is a leading cause of respiratory infections in infants and young children, making the quest for an effective vaccine critical. Early attempts to create a vaccine encountered setbacks, including safety concerns and insufficient efficacy.
Initial efforts to develop a live attenuated RSV vaccine began in the 1960s. Researchers focused on altering the virus to provoke a strong immune response without causing disease. This process involved multiple viral passage techniques, which aimed to weaken the virus. Despite promising approaches, these early vaccines often led to enhanced respiratory disease in subsequently infected children, highlighting the need for a more refined vaccine strategy.
In recent years, advancements in molecular biology and virology have spurred renewed interest in live RSV vaccines. Scientists have been able to identify key components of the virus that can be targeted to improve vaccine safety and efficacy. Current research is focused on optimizing the vaccine’s ability to stimulate a robust immune response, while minimizing the risk of adverse effects. The ongoing studies aim to provide a much-needed tool to combat RSV infections effectively.
Effectiveness of the live RSV vaccine
The live RSV vaccine has shown promise in clinical trials and studies conducted in recent years. Its effectiveness is evaluated based on its ability to stimulate a strong immune response against respiratory syncytial virus (RSV), which is a major cause of respiratory illnesses in infants and young children.
Several studies have demonstrated that this live vaccine can significantly reduce the incidence of RSV infections. For instance, a clinical trial conducted with a diverse population of infants showed that those who received the live RSV vaccine had a 65% lower rate of RSV-related hospitalizations compared to the control group.
In addition to reducing the overall incidence, the vaccine also appears to lessen the severity of RSV-related symptoms. While more research is still needed to fully understand its long-term effectiveness and potential for broader use, the live RSV vaccine represents a critical advancement in the fight against RSV, particularly for vulnerable populations such as premature infants.
Side effects of the live RSV vaccine
The live RSV vaccine has shown great promise in providing immunity against respiratory syncytial virus (RSV). However, like any vaccine, it is important to consider the side effects that may arise. Understanding these effects helps to prepare for what may occur post-vaccination and how to respond effectively.
- Fever
- Mild rash
- Swelling or pain at the injection site
- Fatigue
In some cases, individuals may experience more serious reactions, although these are rare. It’s crucial to monitor for symptoms such as difficulty breathing, high fever, or severe allergic reactions. Anyone who shows these symptoms should seek immediate medical attention. The benefits of vaccination must be weighed against these potential risks, and discussing any concerns with a healthcare provider is highly advisable before vaccination.
Future prospects for the live RSV vaccine
The future prospects for the live RSV vaccine are promising, with ongoing research and development focusing on enhancing its effectiveness and safety. As respiratory syncytial virus (RSV) continues to pose a significant health risk, especially among infants and the elderly, scientists are working diligently to create vaccines that can provide long-lasting immunity.
Studies are currently underway to evaluate various formulations and delivery methods. A major aspect of this research involves understanding how to produce a strong immune response with minimal side effects. There are also efforts to explore the use of novel adjuvants that can enhance vaccine responses. This could lead to more robust protection against RSV without the drawbacks associated with traditional vaccine components.
Moreover, collaboration between pharmaceutical companies and research institutions is on the rise, which could accelerate the development timeline for the live RSV vaccine. Initiatives aimed at global health equity are also being prioritized, ensuring that once successful, the vaccine will be accessible to populations at risk worldwide. The groundwork laid today will be essential for introducing an effective live RSV vaccine
Frequently Asked Questions
What is RSV and why is it significant?
RSV, or Respiratory Syncytial Virus, is a common virus that causes respiratory infections, particularly in infants and young children. It can lead to severe bronchiolitis and pneumonia in vulnerable populations.
Is the RSV vaccine live or inactivated?
The current RSV vaccines in development are primarily based on recombinant technologies or subunit vaccines, rather than traditional live attenuated viruses. This means they do not contain live virus.
Who are the primary targets for the RSV vaccine?
The primary targets for the RSV vaccine are infants, older adults, and individuals with compromised immune systems, as they are at higher risk for severe RSV infections.
What stage are RSV vaccines currently in?
As of now, several RSV vaccines are in various stages of clinical trials, with some nearing approval. However, no RSV vaccine has been fully approved for general use yet.
What are the expected side effects of the RSV vaccine?
Expected side effects of the RSV vaccine may include mild local reactions at the injection site, fever, and fatigue. Serious side effects are rare.
How does the RSV vaccine help prevent infection?
The RSV vaccine works by stimulating the immune system to recognize and fight off the RSV, thereby reducing the likelihood of infection and severe disease symptoms.
When is the RSV vaccine expected to be widely available?
While specific timelines can vary, health officials anticipate that the RSV vaccine could become available in the next few years, depending on ongoing clinical trial results and regulatory approvals.