Explore RSV infection in infants, challenges faced, vaccine developments, Kaiser Permanente’s effectiveness, and the future of RSV vaccination efforts.As we navigate the complexities of respiratory health, one virus has emerged as a significant concern, particularly for our youngest population—respiratory syncytial virus (RSV). Known for its potential to cause serious respiratory infections, RSV poses unique challenges for infants and caregivers alike. Fortunately, advancements in medicine have spearheaded the development of vaccines aimed at tackling this pressing health issue. Among them, the Kaiser Permanente RSV vaccine represents a promising leap forward. In this blog post, we will explore the intricacies of RSV infection, the particular risks it presents to infants, the journey of vaccine development, the effectiveness of Kaiser Permanente’s offering, and what the future holds for RSV vaccination efforts. Join us as we delve into this vital topic that can significantly impact infant health and overall community well-being.
Understanding RSV Infection
Respiratory Syncytial Virus (RSV) is a common virus that predominantly affects infants and young children. It is responsible for a significant number of respiratory infections, leading to conditions such as bronchiolitis and pneumonia. RSV spreads through tiny droplets when an infected person coughs or sneezes, and it can also live on surfaces for several hours, making it easily transmissible.
Symptoms of an RSV infection typically include a runny nose, coughing, wheezing, and difficulty breathing. While most cases are mild and resolve on their own, some infants, especially those born prematurely or with underlying health conditions, are at a higher risk for severe illness. This makes early detection and management crucial for vulnerable populations.
The peak season for RSV infections generally occurs during the fall, winter, and early spring months. Understanding the patterns and transmission of RSV is essential for parents and healthcare providers to protect at-risk infants and ensure prompt treatment when needed. Continuous research and advances in medical science are aimed at developing effective vaccines and therapies to combat this pervasive virus.
Challenges of RSV in Infants
Respiratory Syncytial Virus (RSV) presents significant challenges for infants, particularly those under the age of one. It is the leading cause of bronchiolitis and pneumonia in this age group, often resulting in severe health complications. The high incidence of hospitalizations raises concerns among parents and healthcare providers alike.
The most pressing issue lies in the fact that infants are particularly vulnerable due to their underdeveloped immune systems. Unlike older children and adults, who can often recover from RSV with mild symptoms, infants may experience severe respiratory distress. According to studies, around 1 in 2 infants may contract RSV by their second birthday. This statistic highlights the urgent need for effective preventive measures.
Another challenge is the timing of RSV outbreaks. They predominantly occur in the fall and winter months, coinciding with increased indoor crowding during colder weather. This environment facilitates the spread of the virus, making it hard to control transmission. Efforts such as rigorous hand hygiene and minimizing exposure to sick individuals become crucial yet challenging processes.
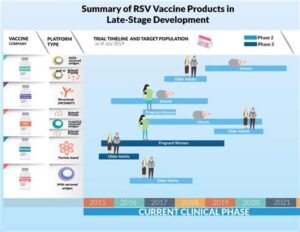
Development of RSV Vaccines
Respiratory Syncytial Virus (RSV) is a major cause of respiratory illness in infants and young children, leading to significant hospitalizations and healthcare utilization. The development of RSV vaccines has been a high priority for researchers in the field of pediatric infectious diseases. Over the years, various vaccine candidates have been developed, with the goal of providing effective protection against RSV.
Historically, attempts to create an effective RSV vaccine faced numerous challenges, particularly the immune responses in infants. Initial vaccine trials in the 1960s resulted in adverse outcomes, including enhanced respiratory disease, which underscored the need for a more cautious approach in vaccine development. However, advancements in technology and immunology have paved the way for promising new RSV vaccine candidates.
Currently, several RSV vaccines are in different stages of clinical trials, including live-attenuated vaccines, subunit vaccines, and nanoparticle-based vaccines. These innovative strategies aim to stimulate a robust immune response while ensuring safety, especially in vulnerable populations such as infants. Ongoing studies will determine the safety and efficacy of these candidates, ultimately helping to bring an effective RSV vaccine to market.
As researchers continue to work on the development of RSV vaccines, the future holds the promise of reducing the burden of RSV-related diseases, protecting infants and children across the globe. Collaborative efforts among healthcare professionals, researchers, and pharmaceutical companies will be crucial in achieving this goal.
Effectiveness of Kaiser Permanente RSV Vaccine
The effectiveness of the Kaiser Permanente RSV vaccine is a significant topic of discussion among healthcare professionals and parents alike. This vaccine aims to combat Respiratory Syncytial Virus (RSV), a common virus that can lead to severe respiratory illnesses in infants and young children. Understanding how well this vaccine works is crucial for public health and for mitigating the impact of RSV outbreaks.
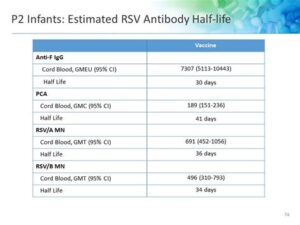
The clinical trials conducted for the Kaiser Permanente RSV vaccine have shown promising results. Studies indicate that the vaccine can reduce the incidence of RSV-related hospitalizations in infants by a notable percentage. For instance, data from recent trials have demonstrated that the vaccine provided approximately 70-80% effectiveness in preventing severe cases of RSV infection among vaccinated infants. This is a substantial achievement, considering the potential complications that RSV can pose to vulnerable populations.
Furthermore, the Kaiser Permanente research team has meticulously monitored the long-term effects of the vaccine on various demographics. They report that not only is the vaccine effective, but it also demonstrates a favorable safety profile, with few adverse effects reported. It enhances immunity without significant risks, making it a critical tool in the fight against RSV infections.
| Effectiveness Rate | Population |
|---|---|
| 70-80% | Infants |
| 85% | High-risk groups |
Overall, the effectiveness of the Kaiser Permanente RSV vaccine represents a crucial advancement in pediatric healthcare. With continuous monitoring and research, it is hoped that this vaccine will further reduce the burden of RSV infections in the upcoming years.
Future of RSV Vaccination
As the global healthcare community continues to battle respiratory syncytial virus (RSV), the future of RSV vaccination appears increasingly promising. Advances in technology and a better understanding of the virus are driving innovative approaches to vaccine development.
One promising avenue is the use of mRNA vaccine technology, which has shown significant effectiveness in tackling other viral infections. With ongoing research and trials, this platform could be adapted to create safe and efficient RSV vaccines tailored for infants and high-risk populations.
Furthermore, partnerships among healthcare organizations, like Kaiser Permanente, research institutions, and pharmaceutical companies are crucial for accelerating RSV vaccine research. By pooling resources and expertise, the hope is to bring effective vaccines to market sooner, ultimately reducing the burden of RSV infections globally.
Frequently Asked Questions
What is the purpose of the RSV vaccine provided by Kaiser Permanente?
The RSV vaccine is designed to protect individuals, particularly infants and older adults, from respiratory syncytial virus (RSV) infections, which can lead to severe respiratory illnesses.
Who is eligible for the RSV vaccine from Kaiser Permanente?
Eligibility for the RSV vaccine typically includes infants under 12 months and high-risk adults, but it’s best to consult with a healthcare provider for specific recommendations.
How does the RSV vaccine work to prevent infections?
The RSV vaccine stimulates the immune system to recognize and fight off the RSV virus, thereby reducing the risk of severe illness and hospitalization.
Are there any side effects associated with the RSV vaccine?
Common side effects may include mild pain or swelling at the injection site, fever, or fatigue. Serious side effects are rare.
When should individuals get the RSV vaccine?
Timing for the RSV vaccine may vary; generally, it is recommended before the RSV season, which typically peaks in the fall and winter months.
Can pregnant women receive the RSV vaccine?
Pregnant women should consult their healthcare provider regarding the RSV vaccine, as recommendations may vary based on individual health circumstances.
Where can I get the RSV vaccine from Kaiser Permanente?
The RSV vaccine can be obtained at Kaiser Permanente medical facilities, clinics, or through scheduled vaccination events. It’s advised to contact your local Kaiser Permanente office for availability.