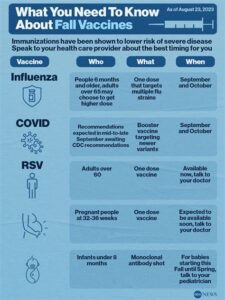
Explore the Kaiser RSV Vaccine’s development, effectiveness, side effects, and future research to understand its impact on respiratory syncytial virus prevention.As respiratory syncytial virus (RSV) continues to pose a significant threat, particularly to infants and the elderly, the development of effective vaccines is crucial. Among the most promising advancements in this field is the Kaiser RSV vaccine, a groundbreaking initiative aimed at combating RSV and its associated complications. In this blog post, we will explore what the Kaiser RSV vaccine is, tracing its development from initial research to clinical trials. We’ll also delve into its effectiveness, discuss potential side effects, and consider the future of RSV vaccine research. Join us as we uncover the science behind this important health innovation and its potential to save lives.
What is Kaiser RSV Vaccine?
The Kaiser RSV Vaccine is a groundbreaking immunization developed to protect against respiratory syncytial virus (RSV), a common virus that primarily affects infants and young children. RSV is a leading cause of respiratory illness in children, often resulting in severe cases that require hospitalization. The development of this vaccine aims to reduce the incidence of RSV infections and their associated complications.
RSV is notorious for causing bronchiolitis and pneumonia, particularly in vulnerable populations, including premature infants and children with underlying health conditions. The Kaiser RSV Vaccine targets the virus by stimulating the immune system to produce antibodies specific to RSV, thereby enhancing the body’s defense mechanisms against infection.
This vaccine represents a significant advancement in pediatric healthcare and has been developed following extensive research and clinical trials. By offering protection against RSV, the vaccine hopes to alleviate the burden on healthcare systems and impr
Development of Kaiser RSV Vaccine
The Kaiser RSV Vaccine represents a crucial advancement in the fight against respiratory syncytial virus (RSV), particularly for vulnerable populations such as infants and older adults. The development of this vaccine is the result of extensive research and collaboration among scientists, pharmaceutical companies, and healthcare organizations.
Initial research on RSV vaccines began several decades ago, but many previous attempts faced significant challenges, including safety concerns and insufficient efficacy. However, recent breakthroughs in vaccine technology and understanding of the virus’s biology have paved the way for new, innovative approaches. The Kaiser Permanente research team focused on developing a subunit vaccine, which uses harmless pieces of the virus to stimulate the immune system without causing the disease.
Clinical trials for the Kaiser RSV Vaccine commenced to assess its safety and effectiveness. These trials included diverse populations to ensure comprehensive data on how the vaccine performs across different demographics. Early results indicate promising efficacy, leading to optimism that the Kaiser RSV Vaccine could significantly reduce hospitalizations and deaths related to RSV.
| Phase | Description |
|---|---|
| Preclinical | Laboratory and animal testing to evaluate safety and immunogenicity. |
| Phase 1 | Initial human trials focused on safety and dosage. |
| Phase 2 | Expanded trials to assess effectiveness and side effects. |
| Phase 3 | Large-scale trials to confirm the efficacy in a broader population. |
As the vaccine progresses through these stages, researchers remain committed to transparent communication about findings and ensuring accessibility for those most in need. The Kaiser RSV Vaccine not only represents a scientific achievement but also embodies hope for millions affected by RSV each year.
Effectiveness of Kaiser RSV Vaccine
The Kaiser RSV Vaccine represents a significant advancement in the fight against Respiratory Syncytial Virus (RSV), a common virus that can lead to severe respiratory illness, especially in infants, the elderly, and those with weakened immune systems. Understanding the effectiveness of this vaccine is crucial for public health, given the high rates of RSV-related hospitalizations each year.
Clinical trials have shown that the Kaiser RSV Vaccine is effective in significantly reducing the incidence of RSV infections among targeted populations. In fact, studies suggest that the vaccine may prevent up to 70% of severe RSV cases. This effectiveness is particularly vital for infants and young children, who are at the highest risk for serious complications due to RSV.
Additionally, the vaccine’s effectiveness extends beyond preventing infections; it also helps in reducing the severity of symptoms in those who do contract RSV. Studies indicate that vaccinated individuals experience milder symptoms and shorter hospitalization times. This critical benefit not only enhances patient outcomes but also alleviates the burden on healthcare systems.
The ongoing monitoring of the Kaiser RSV Vaccine in real-world settings will further illuminate its effectiveness and help identify any specific population groups that may benefit most from vaccination. As more data becomes available, it will be essential to continue assessing the long-term impacts of this vaccine on public health.
Side Effects of Kaiser RSV Vaccine
The Kaiser RSV Vaccine is a promising development in the quest to provide immunity against respiratory syncytial virus (RSV). As with any medical intervention, understanding the side effects is crucial for prospective patients and healthcare providers.
Clinical trials have shown that most individuals experience only mild to moderate side effects.
| Common Side Effects | Frequency |
|---|---|
| Pain at the injection site | Common |
| Fatigue | Common |
| Headache | Occasional |
| Fever | Uncommon |
More serious side effects, though rare, have also been reported. These may include allergic reactions such as rash, itching, or difficulty breathing. It is important for individuals to consult with their healthcare provider if they have a history of severe allergies or previous reactions to vaccines.
Overall, the benefits of receiving the Kaiser RSV Vaccine outweigh the potential risks for most people, especially those in high-risk categories such as infants and the elderly. Continuous monitoring for side effects post-vaccination is essential to ensure the safety and effectiveness of the vaccine.
Future of Kaiser RSV Vaccine Research
The future of Kaiser RSV Vaccine research holds great promise as scientists continue to uncover innovative methods for combating respiratory syncytial virus (RSV). As RSV is known to significantly impact vulnerable populations, particularly infants and the elderly, advanced research initiatives are vital for developing effective and safe vaccines.
One area of focus in future research is enhancing the effectiveness of the Kaiser RSV Vaccine. Researchers are investigating different formulations and delivery mechanisms to ensure robust immune responses. For instance, studies are being conducted on the incorporation of novel adjuvants to boost vaccine efficacy, along with exploring intranasal delivery systems that could provide more localized protection against RSV.
Additionally, long-term safety and monitoring of the vaccine’s effects in diverse populations remain critical. Ongoing clinical trials aim to gather comprehensive data, ensuring that any potential side effects are assessed and managed efficiently. This continuous surveillance will enhance public trust and acceptance of the Kaiser RSV Vaccine, which is essential for achieving widespread vaccination coverage.
Moreover, globalization of vaccine development efforts could pave the way for innovative collaborative projects. Partnerships between different research institutions and pharmaceutical companies could accelerate findings in Kaiser RSV Vaccine studies, ultimately leading to new vaccination strategies and treatments that could be implemented globally.
In conclusion, while there are significant challenges ahead, the future of Kaiser RSV Vaccine research is filled with opportunities that could lead to groundbreaking advancements in the fight against RSV. The ongoing commitment to res
Frequently Asked Questions
What is the Kaiser RSV vaccine?
The Kaiser RSV vaccine is a vaccine developed to protect against respiratory syncytial virus (RSV), which is a common virus that can cause respiratory infections, especially in young children and the elderly.
Who is the target demographic for the Kaiser RSV vaccine?
The primary target demographic for the Kaiser RSV vaccine includes infants and young children, as well as older adults who are at greater risk for severe RSV infections.
What are the potential benefits of the Kaiser RSV vaccine?
The potential benefits of the Kaiser RSV vaccine include reducing the incidence of RSV-related hospitalizations and complications, lowering healthcare costs, and providing increased protection for vulnerable populations.
Is the Kaiser RSV vaccine safe?
Clinical trials have been conducted to assess the safety of the Kaiser RSV vaccine, and preliminary results suggest it is safe with manageable side effects, similar to other vaccines.
When is the Kaiser RSV vaccine expected to be available?
The availability of the Kaiser RSV vaccine will depend on the results of ongoing clinical trials and regulatory approvals, but it is anticipated to be available for widespread use in the coming years.
How does the Kaiser RSV vaccine work?
The Kaiser RSV vaccine works by stimulating the immune system to produce antibodies against RSV, helping to prevent infection and reduce the severity of symptoms if infection occurs.
Are there any alternatives to the Kaiser RSV vaccine?
Yes, there are other preventive measures and treatments for RSV, including monoclonal antibodies like palivizumab, which can help protect high-risk infants from severe RSV disease.