Explore the Kaiser RSV vaccine, its availability factors, production efforts, expanded access, and future prospects in this informative guide.As the battle against respiratory syncytial virus (RSV) continues, the Kaiser RSV vaccine has emerged as a promising line of defense, particularly for vulnerable populations. This blog post delves into the intricacies surrounding the availability of this crucial vaccine, shedding light on what it is, the factors that influence its supply, and the ongoing efforts to boost production. With RSV posing significant health risks, especially to infants and older adults, understanding the current landscape of vaccine accessibility is vital. We will explore the steps being taken to expand access to the Kaiser RSV vaccine and what the future holds for its distribution. Join us as we navigate through these essential topics to better comprehend how the Kaiser RSV vaccine is making strides in public health.
Understanding Kaiser RSV Vaccine
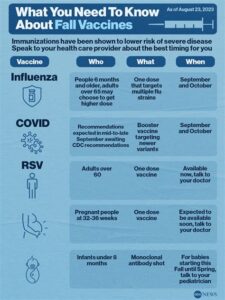
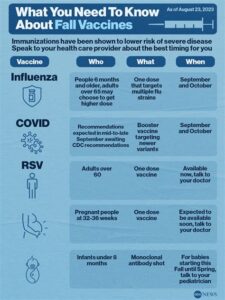
The Kaiser RSV Vaccine is a crucial advancement in the fight against Respiratory Syncytial Virus (RSV), which poses significant health risks, particularly to infants and the elderly. Developed to provide immunity against RSV, this vaccine aims to reduce hospitalization rates and alleviate the burden on healthcare systems.
RSV is a common virus that can lead to severe respiratory illness, and while most individuals recover within a week or two, the virus can be particularly dangerous for high-risk groups. The Kaiser RSV Vaccine works by stimulating the immune system to recognize and combat the virus effectively, potentially leading to a significant decrease in incidence rates among vulnerable populations.
As the demand for vaccines continues to rise due to the increasing awareness of RSV’s consequences, understanding the Kaiser RSV Vaccine’s formulation, efficacy, and distribution becomes essential. Ongoing studies aim to ensure that the vaccine is both safe and effective, with re
Factors Impacting Vaccine Availability
The availability of theKaiser RSV vaccine is influenced by a multitude of factors that play a crucial role in ensuring that this important immunization reaches those who need it most. Understanding these factors is essential for grasping the broader context of vaccine distribution, particularly in light of current health challenges.
One of the primary factors impacting availability is the production capacity of the vaccine itself. Pharmaceutical manufacturers must ensure that they can meet the demand for the vaccine, which can fluctuate based on seasonal outbreaks of Respiratory Syncytial Virus (RSV). Additionally, any disruptions in the supply chain can hinder the timely distribution of the vaccine.
Another important aspect is the regulatory approval process. Before the Kaiser RSV vaccine can be rolled out to the public, it must undergo rigorous testing and receive approval from health authorities. This process can be time-consuming, and delays can significantly affect availability.
Furthermore, the distribution networks and logistical challenges also play a role in how quickly and effectively the vaccine can be delivered to healthcare facilities. Vaccines must be stored and transported under specific conditions to maintain their efficacy, making efficient logistics essential for ensuring that the vaccine is accessible to all who need it.
Ultimately, a combination of production, regulatory, and logistical factors influences the overall availability of the Kaiser RSV vaccine, highlighting the complexity involved in vaccine distribution.
Efforts to Increase Vaccine Production
The production and distribution of the Kaiser RSV vaccine is crucial to addressing respiratory syncytial virus (RSV) challenges, particularly for vulnerable populations such as infants and the elderly. To ensure that there is an adequate supply of this life-saving vaccine, several key efforts are underway.
Firstly, pharmaceutical companies are collaborating with government agencies to optimize manufacturing processes. This includes investments in state-of-the-art technology and facilities, which help accelerate the production timeline and enhance efficiency. With these advancements, companies can produce higher volumes of the Kaiser RSV vaccine without compromising safety and efficacy.
Furthermore, regulatory agencies are streamlining approval processes for production facilities and methods. By facilitating quicker reviews and approvals, they aim to reduce bottlenecks that traditionally slow down vaccine availability. This regulatory support is essential for meeting the increasing demand as RSV cases rise seasonally.
| Efforts to Increase Vaccine Production | Description |
|---|---|
| Collaboration with Government Agencies | Working together to improve manufacturing processes and speed up production. |
| Investment in Technology | Utilizing advanced technology to enhance production capacity and efficiency. |
| Streamlining Regulatory Approvals | Reducing bottlenecks in the approval process to enhance availability. |
Through these combined efforts, the aim is to achieve widespread availability of the Kaiser RSV vaccine, ultimately e
Expanding Access to the Kaiser RSV Vaccine
The expansion of access to the Kaiser RSV Vaccine is crucial in combating the respiratory syncytial virus (RSV) among vulnerable populations, particularly infants and older adults. As the vaccine becomes available, it is essential to ensure that those who need it the most can easily obtain it. This expansion involves multiple strategies that aim to enhance accessibility across various regions and communities.
One of the primary factors in expanding access is the establishment of partnerships with healthcare providers, community organizations, and public health agencies. These collaborations can facilitate outreach programs that educate communities about the vaccine, emphasizing its importance and safety. By increasing public awareness, more individuals will be informed about the option of receiving the Kaiser RSV Vaccine.
Moreover, improving logistical aspects such as distribution networks and infrastructure is vital. Mobile vaccination clinics, for instance, can bring the vaccine directly to underserved areas, eliminating transportation barriers. Moreover, subsidies or financial assistance programs may also be necessary to reduce the cost of the vaccine, thereby increasing its affordability for low-income families. Ultimately, these efforts will play an essential role in ensuring that the Kaiser RSV Vaccine is widely accessible, protecting those at highest risk of severe illness from RSV.
Future of Kaiser RSV Vaccine Availability
The future of Kaiser RSV vaccine availability is a topic of significant importance as global health organizations and pharmaceutical companies continue to work diligently to combat Respiratory Syncytial Virus (RSV). With the rising incidence of RSV infections, particularly among vulnerable populations such as infants and the elderly, the demand for effective vaccination has never been more critical. The Kaiser RSV vaccine stands as a beacon of hope in the effort to mitigate the impact of this virus.
Several factors will influence the future availability of the Kaiser RSV vaccine. This includes advancements in vaccine technology, regulatory approvals, and the capacity for large-scale production. The collaboration between public health entities, researchers, and manufacturers will play a pivotal role in ensuring that doses can be distributed efficiently to those most in need.
Moreover, as we look ahead, public awareness and community outreach will be essential in driving the uptake of the Kaiser RSV vaccine. Educational campaigns will help demystify the vaccine process and emphasize the importance of timely vaccination for at-risk populations. Together, these efforts will pave the way for enhanced availability of the Kaiser R
Frequently Asked Questions
What is the Kaiser RSV vaccine?
The Kaiser RSV vaccine is designed to protect against respiratory syncytial virus (RSV), which can cause severe respiratory infections, particularly in infants and older adults.
Who is eligible to receive the Kaiser RSV vaccine?
Eligibility for the Kaiser RSV vaccine primarily includes infants, young children, and high-risk older adults, although specific recommendations may vary.
When will the Kaiser RSV vaccine be available?
The availability of the Kaiser RSV vaccine depends on regulatory approval and distribution plans, but it is anticipated to be rolled out in the fall.
How can one get the Kaiser RSV vaccine?
Individuals can receive the Kaiser RSV vaccine through healthcare providers or designated vaccination clinics, often requiring an appointment.
What are the benefits of the Kaiser RSV vaccine?
The Kaiser RSV vaccine aims to significantly reduce the incidence and severity of RSV infections, thereby protecting vulnerable populations.
Are there any side effects associated with the Kaiser RSV vaccine?
Like any vaccine, the Kaiser RSV vaccine may cause side effects, such as mild pain at the injection site, fever, or fatigue, but serious side effects are rare.
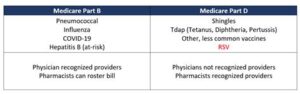
Is the Kaiser RSV vaccine covered by insurance?
Many insurance plans cover vaccines, including the Kaiser RSV vaccine; however, it’s recommended to check with your specific insurance provider for details.