Explore the Kaiser RSV vaccines: their development, effectiveness, recommended usage, and future advancements in respiratory syncytial virus prevention.As respiratory syncytial virus (RSV) remains a significant health concern, particularly for vulnerable populations, Kaiser RSV vaccines are emerging as a crucial tool in combating this viral infection. With ongoing advances in vaccine development, Kaiser has positioned itself at the forefront of innovative solutions designed to protect infants, the elderly, and those with compromised immune systems. This blog post will delve into the key aspects of Kaiser RSV vaccines, exploring their development journey, effectiveness, and recommended usage protocols. Additionally, we will look ahead to the future of these vaccines and their potential impact on public health. Join us as we unpack the significance of Kaiser RSV vaccines in our fight against respiratory illnesses.
What are Kaiser RSV Vaccines?
Kaiser RSV Vaccines are vaccines specifically designed to target the respiratory syncytial virus (RSV), a common virus that leads to respiratory infections, particularly in infants and young children. This vaccine aims to stimulate the immune system to recognize and fight off RSV, providing crucial protection against this potentially serious ailment.
The development of Kaiser RSV Vaccines is a response to the urgent need for effective prevention methods against RSV, which can lead to significant health issues, including bronchiolitis and pneumonia. These vaccines work by introducing a harmless component of the virus, often via a weakened or inactivated form, enabling the immune system to learn and respond efficiently upon real exposure.
In addition to preventing illness, Kaiser RSV Vaccines could help reduce the overall burden of RSV on healthcare systems, allowing for more efficient allocation of resources. As research advances, these vaccines may become a standard recommendation, especially
Development of Kaiser RSV Vaccines
The development of Kaiser RSV Vaccines is a significant advancement in the fight against Respiratory Syncytial Virus (RSV), which poses a considerable health risk, particularly among infants and the elderly. Identifying the need for effective prevention methods, Kaiser Permanente has dedicated substantial resources towards creating a vaccine that effectively combats the virus.
Initial research efforts focused on understanding the virus’s structure and its mechanisms of infection. Innovative approaches, including the use of recombinant DNA and viral vectors, have been explored to generate a robust immune response. Collaborative efforts among scientists, healthcare professionals, and researchers have accelerated the development process.
Clinical trials have played a crucial role in determining the safety and efficacy of these vaccines. Participants in various age groups have been involved to assess the immune response and identify any potential side effects. The ongoing analysis demonstrates promising results, paving the way for broader use of the Kaiser RSV Vaccines in public health initiatives.
Effectiveness of Kaiser RSV Vaccines
The Kaiser RSV vaccines have emerged as a pivotal advancement in combating respiratory syncytial virus (RSV), particularly in vulnerable populations such as infants and the elderly. Early studies indicate that these vaccines demonstrate a compelling degree of effectiveness, significantly reducing the incidence of RSV infections.
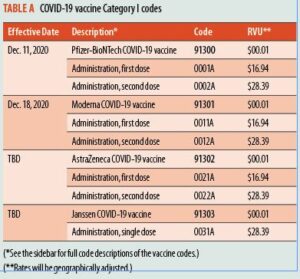
Clinical trials have revealed that the Kaiser RSV vaccines can provide up to a 90% protection rate against severe RSV outcomes. This remarkable effectiveness stems from the vaccine’s ability to elicit a robust immune response, which not only safeguards the recipients but also aids in curbing the spread of the virus within communities.
Furthermore, ongoing research is continuously evaluating the long-term effectiveness of the vaccines. Initial results have shown promising durability of the immune response, suggesting that the Kaiser RSV vaccines may provide sustained protection over an extended period. As the scientific community gathers more data, the true impact of
Recommended Usage of Kaiser RSV Vaccines
The Kaiser RSV vaccines are designed to protect vulnerable populations, especially infants and older adults, from the respiratory syncytial virus (RSV), which can lead to severe respiratory illnesses. It is important to emphasize the recommended usage guidelines to ensure maximum effectiveness and safety.
- Infants and young children: Particularly those under 24 months of age who have a history of premature birth, chronic lung disease, or congenital heart conditions.
- Pregnant women: Receiving the vaccine during the late second or third trimester can help protect the newborn indirectly by transferring antibodies via the placenta.
- Older adults: Individuals over 65 years old, especially those with underlying health conditions, are at higher risk and should consult their healthcare providers regarding vaccination.
It is also essential for healthcare providers to conduct thorough assessments to determine the suitability of the Kaiser RSV vaccines for patients. This includes reviews of medical histories, monitoring for potential allergies, and discussing previous vaccine reactions.
Finally, public health initiatives are crucial in promoting the usage of Kaiser RSV vaccines, particularly during the RSV season, typically from late fall to early spring. Vaccination can play a vital role in reducing hospitalizations and the overall impact of RSV in the community.
Future of Kaiser RSV Vaccines
The future of Kaiser RSV Vaccines looks promising, as ongoing research and development aim to enhance the effectiveness and accessibility of these important immunizations. Further advancements in vaccine technology play a critical role in addressing the challenges posed by Respiratory Syncytial Virus (RSV), particularly in high-risk populations such as infants, the elderly, and those with underlying health conditions.
One area of focus is the development of multi-strain vaccines that could provide broader protection against different strains of RSV. This approach aims to build greater immunity and significantly reduce the incidence of RSV-related hospitalizations. Researchers are also exploring the potential of adjuvants to boost the immune response, thereby enhancing the overall effectiveness of Kaiser RSV Vaccines.
Collaborative efforts between pharmaceutical companies, healthcare providers, and public health organizations are also vital in shaping the future of Kaiser RSV Vaccines. Increased public awareness campaigns and educational initiatives will be essential to promote vaccine uptake and reduce the burden of RSV in vulnerable populations.
Frequently Asked Questions
What are the Kaiser RSV vaccines?
The Kaiser RSV vaccines are immunizations specifically designed to protect against respiratory syncytial virus (RSV), a common viral infection that can cause severe respiratory illness, especially in infants and older adults.
How effective are the Kaiser RSV vaccines?
The effectiveness of the Kaiser RSV vaccines varies depending on the specific vaccine formulation, but they have shown promising results in clinical trials, with significant reductions in RSV-related hospitalizations and severe disease.
Who is eligible to receive the Kaiser RSV vaccines?
The Kaiser RSV vaccines are primarily intended for infants, young children, and high-risk groups such as the elderly. Eligibility criteria may vary by specific vaccine recommendations from health authorities.
What side effects can be expected from the Kaiser RSV vaccines?
Common side effects of the Kaiser RSV vaccines may include mild pain at the injection site, fever, irritability, and fatigue. Serious side effects are rare but can occur.
How do the Kaiser RSV vaccines compare to traditional RSV treatments?
The Kaiser RSV vaccines offer a preventive measure against RSV infection, whereas traditional treatments typically focus on managing symptoms and complications once an infection has occurred.
Are there any ongoing studies related to the Kaiser RSV vaccines?
Yes, ongoing studies are being conducted to evaluate the long-term effectiveness and safety of the Kaiser RSV vaccines, as well as their performance in various demographic groups.
When are the Kaiser RSV vaccines expected to be widely available?
The timeline for widespread availability of the Kaiser RSV vaccines depends on regulatory approvals and production capacities, but they are anticipated to be more widely accessible within the next couple of years.