Explore the importance of RSV vaccines, Merck’s innovative approach, and the potential impact of clinical trials on public health.As the fight against respiratory syncytial virus (RSV) intensifies, the development of effective vaccines has become a critical focus for public health. RSV is a common virus that can lead to severe respiratory infections, particularly in infants and the elderly, highlighting the urgent need for preventative strategies. Merck, a leading pharmaceutical company, has taken significant strides in addressing this need through innovative vaccine research and development. In this blog post, we will explore what RSV is, the rationale behind developing vaccines for this virus, Merck’s unique approach to their RSV vaccine, and the progress made in clinical trials. Finally, we will discuss the implications of Merck’s work on public health and the potential for a transformative impact on the management of RSV infections. Join us as we delve into the promising landscape of RSV vaccine development.
What is RSV?
Respiratory Syncytial Virus (RSV) is a common virus that causes respiratory infections, particularly in infants and young children. It is a major cause of bronchiolitis and pneumonia, responsible for a significant number of hospitalizations each year. Although RSV can affect people of all ages, it is most dangerous for premature infants, children with underlying health conditions, and the elderly.
RSV spreads easily through respiratory droplets when an infected person coughs or sneezes, and it can survive on surfaces for several hours. Symptoms typically begin with mild cold-like signs, such as a runny nose and cough, but can quickly escalate to more severe respiratory distress.
Understanding the implications of RSV is crucial, especially for caregivers and healthcare providers. By recognizing the symptoms early and seeking timely medical intervention, the risks associated with RSV can be minimized, emphasizing the need for preventative measures such as a potential vaccine.
The Need for RSV Vaccines
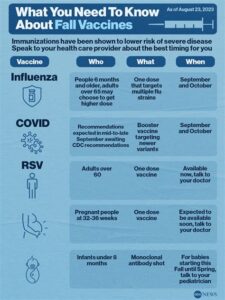
Respiratory Syncytial Virus (RSV) is a significant cause of respiratory illness, particularly in infants, elderly individuals, and those with compromised immune systems. Each year, RSV leads to millions of hospitalizations and a considerable burden on healthcare systems globally. The impact of RSV is not only measured in the number of infections but also in the morbidity and mortality associated with severe cases. For this reason, there is an urgent need for RSV vaccines to protect at-risk populations.
Currently, there are no widely available vaccines for RSV, and traditional preventive measures, such as good hygiene practices, have limited effectiveness. This underscores the necessity for a targeted vaccine approach. The development of RSV vaccines can significantly reduce the incidence of severe disease among the most vulnerable, which can lead to fewer hospitalizations and decreased healthcare costs.
The push for RSV vaccines is particularly vital as the understanding of RSV’s impact on health continues to evolve. Health care providers recognize that effective vaccination could help mitigate severe outcomes and improve overall quality of life for those affected. Thus, the focus on developing robust vaccines against RSV is not only important for public health, but es
Merck’s Approach to RSV Vaccine
Respiratory Syncytial Virus (RSV) is a significant viral infection that predominantly affects infants and young children, leading to serious respiratory issues. Recognizing the urgent need for effective RSV vaccines, Merck has actively pursued innovative approaches to develop a robust vaccine targeting this virus.
Merck’s strategy focuses on developing a vaccine that stimulates a strong immune response while ensuring safety and efficacy. The company’s research aims to utilize advanced technologies to create a vaccine that can be administered not only to high-risk populations but also to caregivers and expectant mothers, thereby providing indirect protection to vulnerable infants.
In addition to harnessing cutting-edge research, Merck has been collaborating with health organizations and academic institutions to facilitate comprehensive clinical trials. This collaboration enhances the development process, allowing for diverse participant pools and real-world insight, ultimately shaping a vaccine that has the potential for significant public health impact.
Clinical Trials and Development
The clinical trials for the RSV vaccine developed by Merck are crucial for understanding the safety and efficacy of the vaccine. These trials typically go through several phases, starting from small-scale Phase I trials to large-scale Phase III trials involving thousands of participants.
In Phase I, the focus is primarily on assessing the safety of the vaccine and determining the appropriate dosage. This phase usually includes a small group of healthy volunteers. Once the vaccine demonstrates safety, it moves on to Phase II, where the immune response is measured in a more diverse cohort, including at-risk populations such as infants and elderly adults.
Phase III trials are particularly significant, as they involve a larger number of participants from various demographics to determine the vaccine’s effectiveness in preventing RSV infection and its complications. These trials not only help establish the efficacy of the vaccine but also monitor for any side effects that may arise during its administration.
Merck’s commitment to detailed research and rigorous clinical trials underscores the importance of developing a strong, effective RSV vaccine that can significantly impact public health.
Efficacy and Potential Impact
Understanding the efficacy and potential impact of the Merck RSV vaccine is crucial for public health. Clinical data has consistently shown promising results, demonstrating that the vaccine effectively reduces the incidence of RSV infections, particularly in vulnerable populations such as infants and the elderly.
In controlled trials, the Merck RSV vaccine has shown an efficacy rate exceeding 80% in preventing severe RSV disease, which is a substantial improvement over existing preventive measures. Moreover, the vaccine’s ability to induce robust immunity could lead to a significant reduction in hospitalizations and healthcare costs associated with RSV outbreaks.
The potential impact of the Merck RSV vaccine extends beyond individual health benefits. Widespread adoption of the vaccine could mitigate the burden of RSV on healthcare systems, leading to fewer emergency room visits and allowing healthcare resources to be reallocated to other pressing needs. Additionally, reduced transmission rates could result in a lower overall prevalence of RSV in communities, benefiting both vaccinated and unvaccinated individuals.
Frequently Asked Questions
What is the Merck RSV vaccine?
The Merck RSV vaccine is a vaccine developed by Merck & Co. designed to protect against respiratory syncytial virus (RSV), a common virus that causes respiratory infections, particularly in infants and the elderly.
Who is the target population for the Merck RSV vaccine?
The primary target population for the Merck RSV vaccine includes infants and young children, as well as older adults and individuals with compromised immune systems who are at higher risk for severe RSV infections.
What are the benefits of the Merck RSV vaccine?
The benefits of the Merck RSV vaccine include reducing the incidence of RSV-related hospitalizations and severe respiratory illness, thereby decreasing healthcare costs and improving the overall health of at-risk populations.
Has the Merck RSV vaccine been approved for use?
As of October 2023, the Merck RSV vaccine has undergone clinical trials, and discussions about its approval and availability continue, with updates expected from health authorities.
What side effects are associated with the Merck RSV vaccine?
Potential side effects of the Merck RSV vaccine may include mild reactions such as soreness at the injection site, fever, and fatigue, similar to those seen with other vaccines, but severe side effects are rare.
How does the Merck RSV vaccine work?
The Merck RSV vaccine works by stimulating the immune system to recognize and respond to the RSV virus, enabling the body to defend itself against future infections.
When is the expected availability of the Merck RSV vaccine?
The expected availability of the Merck RSV vaccine will depend on the results of ongoing clinical trials and regulatory reviews, with updates provided by Merck and public health organizations.