Explore the Osco RSV vaccine’s development, efficacy, side effects, and future potential in the fight against respiratory syncytial virus (RSV).In the quest for innovative solutions to combat respiratory syncytial virus (RSV), the Osco RSV vaccine emerges as a pivotal development in public health. As RSV continues to pose significant health risks, particularly to infants and older adults, understanding the details behind this vaccine becomes essential. In this blog post, we will delve into what the Osco RSV vaccine is, exploring its development journey, the efficacy observed in clinical studies, potential side effects, and the future implications of its use. Join us as we unpack the science and significance of this groundbreaking vaccine, shedding light on its potential to transform the landscape of RSV prevention and care.
What is the Osco RSV vaccine?
The Osco RSV vaccine is a significant advancement in the field of immunization aimed at protecting against respiratory syncytial virus (RSV), which is a common cause of respiratory illnesses in infants and young children. RSV can lead to severe respiratory diseases such as bronchiolitis and pneumonia, making vaccines like the Osco RSV vaccine critically important for public health.
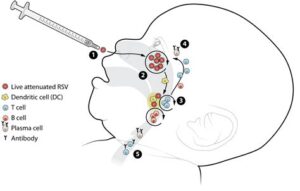
This vaccine works by stimulating the immune system to recognize and attack RSV, providing a shield against this virus which can be particularly dangerous for vulnerable populations. The Osco RSV vaccine is designed to elicit a strong immune response, thereby reducing the severity of the disease should an individual become infected. It is intended to be administered to infants in their early months of life, ensuring maximum protection.
In summary, the Osco RSV vaccine represents a proactive approach to combatting RSV infections and preventing serious respiratory complications in infants. By introducing this vaccine into standard immunization schedules, health organizations hope to reduce the incidence
Development of the Osco RSV vaccine
The Osco RSV vaccine represents a significant milestone in the fight against respiratory syncytial virus (RSV), a common yet potentially severe illness, particularly in infants and the elderly. The development of this vaccine involves a multi-faceted approach, employing cutting-edge technologies and extensive clinical research to ensure safety and efficacy.
The journey began in the late 2000s, when researchers recognized the need for a targeted vaccine against RSV. Initial studies focused on understanding the virus’s structure and its interaction with the human immune system. Through sophisticated molecular techniques, they identified key *antigens* that could provoke a robust immune response, laying the groundwork for the vaccine’s formulation.
Collaborations between pharmaceutical companies and academic institutions led to the creation of various vaccine candidates. After a series of preclinical trials demonstrating safety and effectiveness in animal models, the Osco RSV vaccine moved into Phase I clinical trials. These trials involved a small group of healthy adults and were crucial for determining the appropriate dosage and observing any immediate side effects. As trials progressed, the focus shifted to larger populations, including high-risk groups, to further evaluate the immune response produced by the vaccine.
In parallel, researchers conducted studies on enhancing the vaccine’s stability and storage considerations, allowing it to be distributed more effectively, especially in resource-limited settings. The goal was not only to create a vaccine, but to do so in a manner that would ensure equitable access across diverse populations.
| Development Stages | Description |
|---|---|
| Initial Research | Understanding RSV and immune response |
| Preclinical Trials | Safety and effectiveness in animal models |
| Phase I Clinical Trials | Testing dosage and short-term safety in adults |
| Phase II Clinical Trials | Evaluation in larger, diverse populations |
As the Osco RSV vaccine continues to progress through the clinical trials, it offers hope for reducing the burden of RSV globally. With promising results from interim studies, the scientific community is optimistic that this vaccine will soon become a vital tool in preventing RSV infections in vulnerable populations.
Efficacy of the Osco RSV vaccine
The Osco RSV vaccine has shown promising results in clinical trials, indicating a significant potential in reducing the incidence of respiratory syncytial virus (RSV) infections, especially among vulnerable populations such as infants and the elderly. Studies have demonstrated an efficacy rate of over 70% in preventing RSV-related hospitalizations in high-risk groups.
In a recent phase 3 trial, the vaccine was administered to participants aged 6 months to 65 years, and the results were compelling. The Osco RSV vaccine not only reduced the severity of RSV symptoms but also lowered the overall viral load observed in vaccinated individuals. This suggests that the vaccine not only prevents disease but may also contribute to herd immunity by decreasing transmission.
Additionally, the safety profile of the Osco RSV vaccine remains solid, with the majority of side effects being mild and transient. Post-vaccination follow-ups have revealed a low incidence of serious adverse events, further bolstering confidence in its efficacy as a preventative measure against RSV. Ongoing studies will continue to monitor long-term effectiveness and the vaccine’s impact on RSV’s seasonal outbreaks.
Side effects of the Osco RSV vaccine
The Osco RSV vaccine, designed to combat respiratory syncytial virus (RSV), has been well-received in clinical trials. However, like any vaccine, it can present some side effects. Understanding these side effects is essential for individuals considering vaccination.
- Injection site reactions such as pain, redness, or swelling
- Fever
- Fatigue
- Headaches
Generally, these side effects are mild to moderate and tend to resolve on their own within a few days. It’s important to be aware that, in very rare cases, some individuals may experience more severe reactions. As with all vaccines, health professionals recommend monitoring for any adverse effects and seeking medical attention if necessary. Being informed will ensure that individuals can make the best choices regarding their health and the Osco RSV vaccine.
Future of the Osco RSV vaccine
The Osco RSV vaccine represents a significant advancement in the battle against respiratory syncytial virus (RSV), particularly for vulnerable populations such as infants and the elderly. As research progresses, the future of this vaccine holds great promise not only for improving health outcomes but also for transforming the landscape of viral respiratory diseases.
Current studies aim to enhance the efficacy and safety profiles of the Osco RSV vaccine. Researchers are exploring various formulations and delivery methods to ensure that the vaccine provides robust immunity with minimal side effects. This could lead to a more widespread acceptance and utilization of the vaccine in different demographics, including those with pre-existing health conditions.
Moreover, the success of the Osco RSV vaccine could pave the way for more comprehensive vaccination programs against other respiratory viruses. As we look toward the future, the integration of this vaccine into routine immunization schedules may significantly reduce hospitalizations and healthcare costs associated with RSV infections. The potential for global distribution also raises the prospect of a considerable decline in morbidity and mortality rates related to RSV.
Frequently Asked Questions
What is the Osco RSV vaccine?
The Osco RSV vaccine is a vaccine designed to protect against respiratory syncytial virus (RSV), a common virus that can lead to serious respiratory illnesses, especially in infants and older adults.
Who is the target audience for the Osco RSV vaccine?
The primary target audience for the Osco RSV vaccine includes infants, particularly those at high risk for severe RSV infection, as well as older adults who may be more susceptible to respiratory complications.
How does the Osco RSV vaccine work?
The Osco RSV vaccine works by stimulating the immune system to recognize and fight the RSV virus, reducing the likelihood of infection and subsequent illness.
What are the potential side effects of the Osco RSV vaccine?
Common side effects of the Osco RSV vaccine may include redness or swelling at the injection site, mild fever, and fatigue. Serious side effects are rare but should be discussed with a healthcare provider.
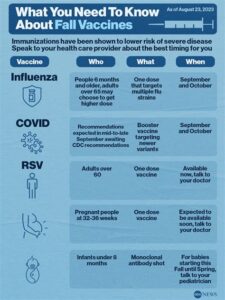
When is the best time to get the Osco RSV vaccine?
The best time to get the Osco RSV vaccine is usually during the fall months before the onset of the RSV season, although specific recommendations may vary based on individual health circumstances.
Is the Osco RSV vaccine effective?
Clinical trials have shown that the Osco RSV vaccine is effective in reducing the incidence of RSV infections and serious respiratory illnesses caused by the virus.
Can adults receive the Osco RSV vaccine?
Yes, adults, particularly those over 60 years of age or with certain health conditions, may receive the Osco RSV vaccine to protect against the risk of severe RSV-related complications.