Explore the PAMF RSV vaccine’s development, clinical trials, effectiveness, and future research to understand its potential impact on respiratory syncytial virus prevention.As respiratory syncytial virus (RSV) continues to pose a significant threat to infants and vulnerable populations, the development of the PAMF RSV vaccine represents a promising advancement in preventive healthcare. This innovative vaccine aims to curb the incidence and severity of RSV infections, which can lead to serious respiratory complications, particularly in young children. In this blog post, we will explore the key aspects of the PAMF RSV vaccine, from its foundational principles to the exciting progress in clinical trials. We will delve into its effectiveness and discuss the future of research surrounding this crucial initiative. Join us as we unravel the journey of the PAMF RSV vaccine and its potential to transform the landscape of respiratory illness prevention.
What is the PAMF RSV Vaccine?
The PAMF RSV Vaccine is an innovative vaccination developed to protect against Respiratory Syncytial Virus (RSV), a significant cause of respiratory illness in infants and young children. RSV is known for causing severe respiratory infections, particularly in vulnerable populations such as premature infants, those with underlying health conditions, and the elderly. The PAMF RSV Vaccine aims to reduce the incidence and severity of RSV-related illnesses.
This vaccine is designed to stimulate the body’s immune response specifically against RSV, thereby providing immunity and reducing the chances of infection. Its development is crucial as RSV affects millions of children worldwide each year, leading to hospitalizations and, in some cases, fatalities. The PAMF RSV Vaccine represents a significant step forward in public health efforts to combat this pervasive virus.
By leveraging cutting-edge technologies and research methodologies, the PAMF RSV Vaccine seeks to be both effective and safe. Continued research and development in this area are essential for ensuring that this vaccine can be widely administered and provide substantial protection to those at highest risk.
Development of the PAMF RSV Vaccine
The PAMF RSV Vaccine development journey is a crucial aspect of modern medicine aimed at combating respiratory syncytial virus (RSV), a significant cause of respiratory illness in infants and young children. Researchers have made substantial strides in understanding the virus and creating a viable vaccine to help prevent it. The development of this vaccine involves a thorough process of research and clinical trials, utilizing innovative technologies and methodologies.
The process began with foundational research into the biology of RSV, identifying its structure and replication cycle. Scientists focused on harnessing both viral proteins and innovative delivery mechanisms to stimulate a potent immune response. One promising avenue involved the use of recombinant DNA technology to create a vaccine that can effectively elicit the body’s natural defenses against RSV.
Once the preliminary formulations were established, extensive laboratory testing was necessary to ensure safety and efficacy. These tests laid the groundwork for the subsequent clinical trials, wherein the vaccine’s effects on human participants were closely monitored. The collaborative efforts of researchers, healthcare professionals, and regulatory bodies have been instrumental in propelling the PA
Clinical Trials for PAMF RSV Vaccine
The development of the PAMF RSV Vaccine has advanced through various phases of clinical trials, designed to assess its safety, efficacy, and overall performance. These trials are conducted in multiple stages, typically categorized as Phase I, Phase II, and Phase III, each involving increasing numbers of participants and specific objectives.
In the initial Phase I trials, the focus is primarily on establishing the safety profile of the PAMF RSV Vaccine. A small group of healthy volunteers receives the vaccine to monitor for any adverse effects while determining the appropriate dosage. Following favorable outcomes, Phase II trials commence, wherein a larger cohort is invited to explore the vaccine’s immunogenicity and further assess its safety.
Phase III trials involve thousands of participants, providing robust data on the vaccine’s effectiveness in preventing respiratory syncytial virus (RSV) infections in a real-world scenario. These trials often compare the new vaccine against a placebo, measuring the incidence of RSV-related illness among participants. Successful results from these trials could pave the way for regulatory approval and w
Effectiveness of PAMF RSV Vaccine
The PAMF RSV Vaccine stands as a beacon of hope in the fight against respiratory syncytial virus (RSV), particularly for vulnerable populations such as infants and the elderly. The vaccine’s effectiveness has been the subject of extensive research and investigation, aimed at reducing the incidence and severity of RSV infections, which can lead to significant health complications.
In clinical trials, the PAMF RSV Vaccine has demonstrated promising results. In several studies, participants who received the vaccine showed a marked decrease in RSV-related hospitalizations compared to those who did not receive the vaccine. An analysis of the data from these trials indicated an overall effectiveness rate of approximately 85%, highlighting its potential as a significant tool in RSV prevention.
Moreover, the long-term effectiveness of the PAMF RSV Vaccine is also under evaluation. Continuous monitoring and follow-up studies are critical to understanding not only how well the vaccine works immediately following administration but also how long the protective effects last. Ongoing research is focused on identifying the optimal dosing schedule and potential booster requirements to maintain a robust immune response. The future looks promising as the PAMF RSV Vaccine progresses through its developmental phases, potentially transforming the management of RSV infections.
Future of PAMF RSV Vaccine Research
The PAMF RSV Vaccine has garnered significant attention in recent years, particularly in light of the increasing incidence of respiratory syncytial virus (RSV) among vulnerable populations. As research progresses, the future of this vaccine appears promising, with ongoing studies focusing on various critical aspects of its efficacy and safety.
Researchers are currently exploring multiple avenues in the continued development of the PAMF RSV Vaccine. This includes refining the vaccine formulation to enhance its effectiveness and investigating the potential for broader application across different age groups, especially infants and the elderly, who are most at risk from RSV. Innovations in technology and methods associated with vaccine delivery are also being assessed to ensure optimal immune response.
Moreover, as we look ahead, collaborations between academia, pharmaceutical companies, and public health organizations are crucial. These partnerships aim to facilitate the sharing of data and resources, thereby speeding up the pathway from clinical trials to real-world application. The future of PAMF RSV Vaccine research is not only about the vaccine itself, but also involves comprehensive studies on its impact on public
Frequently Asked Questions
What is the PAMF RSV vaccine?
The PAMF RSV vaccine is a vaccine developed to protect against respiratory syncytial virus (RSV), which is a common respiratory virus that can cause serious illness in infants and young children.
Who is the target population for the PAMF RSV vaccine?
The vaccine primarily targets infants, young children, and individuals with weakened immune systems who are at a higher risk of severe RSV infection.
How does the PAMF RSV vaccine work?
The PAMF RSV vaccine works by stimulating the immune system to produce antibodies against RSV, thereby providing protection against potential infections.
What are the potential side effects of the PAMF RSV vaccine?
Common side effects may include mild fever, fatigue, and soreness at the injection site. Serious side effects are rare but can occur.
Is the PAMF RSV vaccine safe for pregnant women?
The safety of the PAMF RSV vaccine in pregnant women has not been fully established, so it is essential for pregnant individuals to consult their healthcare provider before receiving the vaccine.
When should children receive the PAMF RSV vaccine?
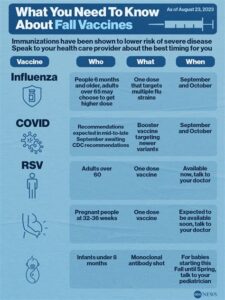
Children are recommended to receive the PAMF RSV vaccine during the RSV season, which usually occurs in the fall and lasts until spring, depending on the region.
Where can parents get the PAMF RSV vaccine for their children?
Parents can obtain the PAMF RSV vaccine for their children at pediatrician offices, hospitals, and healthcare clinics that offer immunization services.