Explore the RSV-IGIV vaccine’s development, benefits, usage in infants, and effectiveness in protecting against respiratory syncytial virus (RSV).Respiratory Syncytial Virus (RSV) is a leading cause of respiratory infections in infants and young children, often resulting in serious health complications. To combat this pervasive virus, the RSV-IGIV vaccine has emerged as a vital tool in pediatric medicine. This blog post delves into the key aspects of the RSV-IGIV vaccine, from its development and benefits to its specific usage in infants and overall effectiveness. Understanding the nuances of this vaccine is crucial for parents, healthcare providers, and caregivers as they navigate the challenges associated with RSV and seek preventative measures to protect vulnerable populations. Join us as we explore the mechanisms behind the RSV-IGIV vaccine and its role in safeguarding the health of our youngest generation.
Understanding the RSV-IGIV Vaccine
The RSV-IGIV vaccine, also known as Respiratory Syncytial Virus Immune Globulin Intravenous, is a specialized immunotherapy designed to help protect vulnerable populations, particularly infants and young children, from Respiratory Syncytial Virus (RSV). RSV is a highly contagious virus that can lead to serious respiratory infections, notably bronchiolitis and pneumonia. Understanding the nuances of this vaccine is crucial for parents, healthcare providers, and caregivers.
Developed from human immunoglobulin, the RSV-IGIV vaccine contains antibodies that are specifically targeted at neutralizing the RSV virus. This makes it particularly effective at reducing the severity of RSV infections in those who are at the highest risk, such as premature infants or those with underlying health conditions. Unlike traditional vaccines, which stimulate the immune system to produce its own antibodies, RSV-IGIV provides passive immunity through the administration of ready-made antibodies.
Administered as an intravenous infusion, the RSV-IGIV vaccine is typically given during the RSV season, which varies by region but generally occurs in the fall and winter months. The timing of administration and the number of doses can depend on the infant’s risk factors and the guidelines provided by healthcare professionals. Continuous research and data collection are essential to optimize the long-term safety and effectiveness of the RSV-IGIV vaccine for infants and to provide parents with the most reliable information.
Development of RSV-IGIV Vaccine
The Respiratory Syncytial Virus (RSV) is a significant cause of respiratory illness, particularly in infants and young children. To combat this, the RSV-IGIV Vaccine was developed as a preventative measure. The journey of this vaccine’s development has undergone rigorous scientific research and clinical trials, which have been essential in ensuring its safety and efficacy.
The development of the RSV-IGIV Vaccine began in the late 20th century when researchers identified the need for a treatment that could provide passive immunity against RSV. The vaccine comprises immunoglobulin harvested from plasma donors who have high levels of antibodies against RSV. This approach allows for immediate immunity, crucial for vulnerable populations, especially infants at risk of severe disease.
Over the years, various studies have shown that the RSV-IGIV Vaccine offers promising results in reducing the incidence of RSV infections in high-risk infants. The rigorous testing and continuous monitoring of its effects have played a vital role in its development, leading to its eventual approval and usage in clinical settings.
Benefits of the RSV-IGIV Vaccine
The RSV-IGIV Vaccine offers several vital benefits, particularly for infants and young children who are at high risk for Respiratory Syncytial Virus (RSV) infection. One of the most significant advantages of this vaccine is its ability to provide *passive immunity*, which helps protect vulnerable populations from severe respiratory illnesses caused by RSV.
Additionally, the RSV-IGIV Vaccine is designed to reduce hospitalizations resulting from RSV-related complications. A study indicated that infants receiving this vaccine had a significantly lower risk of being admitted to the hospital compared to those who did not receive it. This is crucial for preserving *healthcare resources* and reducing the overall burden of RSV in the healthcare system.
Furthermore, the administration of the RSV-IGIV Vaccine can contribute to improved quality of life for families, as it mitigates the fear and stress often associated with RSV infections. Parents can have peace of mind knowing that their infants are afforded an increased level of protection against a virus that is known for its high morbidity and potential for serious complications.
Usage of RSV-IGIV Vaccine in Infants
The RSV-IGIV vaccine plays a crucial role in protecting vulnerable infants from Respiratory Syncytial Virus (RSV), a leading cause of respiratory illness in young children. Infants, especially those born prematurely or with underlying health conditions, are at a higher risk of severe RSV infections. The introduction of the RSV-IGIV vaccine has significantly enhanced the ability to prevent such infections in this sensitive population.
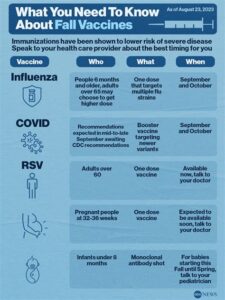
This vaccine is typically administered to infants during their first RSV season, which generally occurs in the fall and winter months. Providing RSV-IGIV can reduce the risk of RSV-related hospitalizations and complications in susceptible infants. The timing and dosage are important factors determined by pediatricians, ensuring that the most vulnerable receive this essential vaccine at the right time.
In clinical practice, the usage of RSV-IGIV vaccine is closely monitored. Health care providers evaluate the infants’ weight and clinical status before administration to optimize the positive effects of the vaccine. This careful approach ensures a high level of safety and efficacy, allowing infants who need it the most to benefit from this preventive measure.
Effectiveness of RSV-IGIV Vaccine
The RSV-IGIV vaccine has been a significant advancement in the prevention of Respiratory Syncytial Virus (RSV) infections, particularly in high-risk infants. This vaccine plays a crucial role in safeguarding vulnerable populations, as RSV can lead to severe respiratory illness in children under two years of age.
Clinical studies have demonstrated that the effectiveness of the RSV-IGIV vaccine in reducing the incidence of RSV-related hospitalizations is remarkably high. Recent trials show that infants who receive this vaccine experience a significant decrease in RSV severity and frequency compared to those who do not receive it. The statistics suggest an effectiveness rate exceeding 70%, which is promising for reducing potential hospital stays and complications arising from RSV infections.
This vaccine works by providing passive immunity, which is especially important for premature infants or those with underlying medical conditions that predispose them to severe RSV disease. The administration of RSV-IGIV not only helps in preventing infections but also provides a crucial window of protection during the peak RSV season, thereby ensuring better health outcomes for the most vulnerable groups.
Frequently Asked Questions
What is the RSV-IGIV vaccine?
The RSV-IGIV vaccine is an immunization designed to protect high-risk infants and children from respiratory syncytial virus (RSV), a common virus that leads to severe respiratory illness.
Who should receive the RSV-IGIV vaccine?
The vaccine is primarily recommended for premature infants, children with certain heart or lung conditions, and other high-risk populations, especially during the RSV season.
How does the RSV-IGIV vaccine work?
The RSV-IGIV vaccine contains antibodies against RSV, which help to bolster the immune system against infection, thereby reducing the severity of the illness if exposure occurs.
What are the possible side effects of the RSV-IGIV vaccine?
Common side effects may include mild fever, irritation at the injection site, and fatigue; however, severe side effects are rare.
When is the best time to administer the RSV-IGIV vaccine?
The vaccine is typically administered shortly before the onset of the RSV season, which usually occurs in fall and peaks in winter.
Can the RSV-IGIV vaccine prevent all RSV infections?
While the RSV-IGIV vaccine significantly reduces the severity of RSV illness, it may not provide complete protection against all strains of the virus.
Is the RSV-IGIV vaccine the same as other vaccines for RSV?
No, the RSV-IGIV vaccine is a specific immunotherapy using immunoglobulin to help high-risk groups, while other RSV vaccines in development target broader populations and may work differently.