Explore the RSV vaccine, its development, clinical trials in Canada, effectiveness, and availability to understand its impact on respiratory health.As respiratory syncytial virus (RSV) continues to pose a significant health risk, particularly for infants and older adults, the development of an effective vaccine is more crucial than ever. The RSV vaccine aims to provide essential protection against this common yet potentially serious virus, and its journey to approval involves an intricate process of research, clinical trials, and rigorous testing. In this blog post, we’ll explore what the RSV vaccine is, delve into its development history, and highlight the ongoing clinical trials being conducted in Canada. Additionally, we will assess the vaccine’s effectiveness and discuss its availability for Canadians. Join us as we navigate the latest advancements in RSV vaccination, shedding light on a key public health initiative that could change the landscape of respiratory infections.
What is the RSV vaccine?
The RSV vaccine is a medical advancement aimed at preventing infection from Respiratory Syncytial Virus (RSV), a virus that causes respiratory disease in infants and young children. RSV is a leading cause of bronchiolitis and pneumonia among children under one year old, making vaccination an essential tool in protecting vulnerable populations.
The vaccine works by stimulating the immune system to recognize and fight off the RSV virus effectively. There are different formulations of the RSV vaccine being developed, with some already undergoing clinical trials. These vaccines are particularly important for infants and those at high risk of severe RSV disease.
In addition to protecting those at risk, the success of the RSV vaccine could lead to significant healthcare savings by reducing the need for hospitalizations, outpatient visits, and long-term respiratory complications associated with RSV infection.
Development of the RSV vaccine
The development of the RSV vaccine has been a long and challenging journey, spanning several decades and involving numerous scientific advances. Respiratory Syncytial Virus (RSV) is a leading cause of respiratory infections in infants and children. Due to its significant health impact, the pursuit of an effective vaccine has been a top priority for researchers worldwide.
Historically, initial attempts to create an RSV vaccine faced setbacks. One of the earliest candidates, developed in the 1960s, caused an enhanced respiratory disease in children who were vaccinated and subsequently infected with the virus. This reinforced the need for a careful approach in vaccine formulation and development.
In recent years, advancements in vaccine technology, such as recombinant proteins and mRNA platforms, have rejuvenated the search for an effective RSV vaccine. Companies like Pfizer and Moderna have made significant strides, with many candidates showing promising results in early clinical trials. Current data indicates a strong potential for a future vaccine that can effectively protect against RSV, particularly for vulnerable populations like infants and the elderly.
Clinical trials in Canada
The respiratory syncytial virus (RSV) has been a significant concern for public health, particularly among infants and the elderly. In Canada, several clinical trials have been conducted to assess the safety and efficacy of the RSV vaccine. These trials are a critical step in bringing the vaccine to market and ensuring it meets the regulatory standards required for approval.
Canadian health authorities and research institutions have taken an active role in the investigation of the RSV vaccine. These trials often involve various populations, including both healthy individuals and those at high risk of severe RSV disease. The trials aim to collect robust data regarding the immune response, potential side effects, and overall effectiveness of the vaccine in preventing RSV infections.
| Trial Phase | Description | Status |
|---|---|---|
| Phase 1 | Initial safety trials to assess the body’s response | Completed |
| Phase 2 | Expanded trials to evaluate dosage and effectiveness | Ongoing |
| Phase 3 | Final trials to confirm efficacy in larger populations | Planned |
Participants in these trials are often closely monitored, and researchers collect valuable data regarding the vaccine’s safety profile. This is paramount in understanding how well the vaccine can perform in real-world scenarios, particularly in Canadian demographics. With ongoing advancements and promising results, clinical trials in Canada play a pivotal role in the future availability of the RSV vaccine to combat this pervasive virus.
Effectiveness of the RSV vaccine
Respiratory Syncytial Virus (RSV) poses a significant health risk, especially to very young children and the elderly. The introduction of the RSV vaccine aims to mitigate this threat. Evaluating the effectiveness of this vaccine is crucial for understanding its impact on public health.
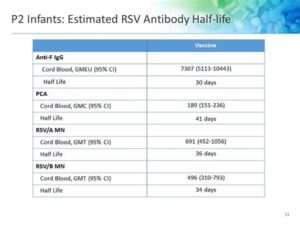
Clinical studies indicate that the RSV vaccine has shown promising efficacy in preventing severe RSV infections. In trials, vaccinated individuals experienced approximately 70-80% reduction in hospitalizations due to RSV, marking a significant achievement in vaccine development.
Furthermore, ongoing monitoring and research continue to assess the long-term effectiveness of the RSV vaccine, including its performance against different strains of the virus. As data accumulates, healthcare professionals will better understand how the vaccine
Availability in Canada
The RSV vaccine is a critical development in the fight against respiratory syncytial virus (RSV), a common virus that can lead to severe respiratory infections, especially in infants and older adults. As of now, the Canadian healthcare system is working diligently to make the RSV vaccine accessible to those at risk.
Currently, health authorities in Canada are evaluating the RSV vaccine options and determining the optimal strategies for vaccine distribution. Several formulations are under consideration, and they aim to target the populations that would benefit most from vaccination. This includes high-risk groups such as infants, elderly individuals, and those with compromised immune systems.
In order to ensure the availability of the RSV vaccine in Canada, public health officials are also focusing on educational campaigns to raise awareness. This involves informing healthcare providers and the general public about the benefits and importance of the RSV vaccine, ultimately fostering a more informed decision-making process regarding vaccination.
Frequently Asked Questions
What is the RSV vaccine?
The RSV vaccine is developed to protect against respiratory syncytial virus (RSV), a common virus that can cause severe respiratory infections, especially in infants and older adults.
Why is the RSV vaccine important in Canada?
In Canada, the RSV vaccine is important because RSV is a leading cause of hospitalization among young children during the winter months and can have serious consequences for at-risk populations.
Who is eligible for the RSV vaccine in Canada?
Eligible individuals typically include infants, particularly those born prematurely or with underlying health conditions, as well as older adults who are at a higher risk of severe illness.
When is the RSV vaccine administered?
The RSV vaccine is usually administered in the fall before the onset of the RSV season, which peaks in the winter months.
What are the side effects of the RSV vaccine?
Common side effects may include mild pain at the injection site, fever, and irritability. Serious side effects are rare.
How effective is the RSV vaccine?
The effectiveness of the RSV vaccine can vary, but clinical trials show it can significantly reduce the severity and incidence of RSV infections in vaccinated populations.
Is the RSV vaccine available in all provinces of Canada?
Availability of the RSV vaccine may vary by province; individuals should consult their healthcare providers or local public health authorities for specific information on access and availability.