Explore the RSV vaccine, informed consent importance, consent form components, potential risks, and how to empower patients with essential information.As the healthcare landscape evolves, the introduction of the RSV vaccine offers a promising advancement in the fight against respiratory syncytial virus, particularly for vulnerable populations. With this progression comes the crucial necessity for informed consent, empowering patients to make knowledgeable decisions regarding their health. In this blog post, we’ll delve into the key aspects of the RSV vaccine, highlighting why understanding its significance is essential. We’ll explore the critical components of the consent form, ensuring clarity on what is involved before vaccination. Furthermore, we’ll address potential risks and side effects, fostering an environment where patients feel confident and well-informed. Join us as we navigate this important aspect of healthcare, emphasizing the importance of patient empowerment through knowledge.
Understanding the RSV Vaccine
The RSV vaccine is designed to protect against Respiratory Syncytial Virus (RSV), a common virus that can lead to serious respiratory illnesses, particularly in infants and older adults. Understanding the importance of the RSV vaccine is crucial, especially as it has been associated with a substantial reduction in hospitalizations and severe cases of RSV infection.
Historically, RSV has been a significant cause of lower respiratory tract infections, leading to substantial morbidity and, in some cases, mortality. The development of the RSV vaccine represented a breakthrough in managing these risks, allowing for more effective prevention strategies to be implemented. It is essential to understand how the vaccine works, its benefits, and why vaccination is recommended.
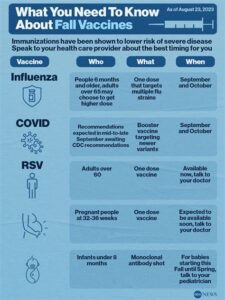
In addition to its protective benefits, the RSV vaccine serves as a critical component of public health initiatives. By vaccinating individuals at high risk—such as premature infants and those with certain health issues—the broader community can achieve herd immunity, which protects even those who cannot be vaccinated. Ultimately, gaining a solid understanding of the RSV vaccine is vital for informed health decisions.
Importance of Informed Consent
Informed consent is a crucial process in the administration of medical treatments, including vaccines like the RSV vaccine. It ensures that patients are fully aware of what they are agreeing to before receiving a medical intervention.
- Respect for Autonomy: Patients have the right to make informed choices regarding their health and treatment options.
- Comprehension: Clear communication about the vaccine, its benefits, and potential risks ensures that patients understand their choices.
- Building Trust: An open dialogue between healthcare providers and patients fosters trust, making patients more likely to adhere to public health recommendations.
Obtaining informed consent means providing comprehensive information regarding the RSV vaccine. This includes details such as the purpose of the vaccine, how it works, and the possible complications that might arise.
Moreover, informed consent can empower patients by facilitating a sense of advocacy regarding their health decisions. It allows them to engage in discussions about their care and treatment options, ensuring they feel confident in their choices.
In conclusion, informed consent is not just a procedural formality; it is an essential component of patient care that respects individuality. It prepares patients to make informed, conscious decisions that align with their health goals when considering the RSV vaccine.
Components of the Consent Form
When it comes to the RSV vaccine, a well-structured consent form is crucial for ensuring that patients and caregivers are thoroughly informed before making a decision. The components of the consent form play an essential role in conveying important information and addressing potential concerns.
| Component | Description |
|---|---|
| Patient Information | The patient’s name, age, and medical history relevant to the vaccine. |
| Vaccine Information | Details about the RSV vaccine, including its purpose, effectiveness, and who should receive it. |
| Benefits | An overview of the potential benefits from receiving the vaccine. |
| Risks and Side Effects | A clear outline of possible adverse reactions and any known side effects associated with the vaccine. |
| Informed Consent | A statement confirming that the patient (or guardian) understands the information presented and agrees to the vaccination. |
| Contact Information | Details for who to contact with questions or concerns post-vaccination. |
The informed consent process not only includes a detailed explanation of the vaccine and its potential implications but also encourages discussion between the healthcare provider and the patient. This allows patients to ask questions and express their concerns, ensuring they fully understand what they are consenting to.
It is imperative that the language used in the consent form is accessible and uncomplicated. This is crucial for patients from various backgrounds to comprehend the information effectively, thus empowering them to make an informed choice regarding their health.
In summary, the components of the consent form for the RSV vaccine are designed to ensure transparency and bolster the informed consent process. A well-prepared form can play a pivotal role in promoting patient understanding and trust in their healthcare providers.
Potential Risks and Side Effects
When considering the RSV vaccine, it is crucial for patients and caregivers to understand the potential risks and side effects associated with it. Like any medical intervention, vaccines can lead to various outcomes, and being informed allows individuals to make educated decisions regarding their health.
The side effects of the RSV vaccine are generally mild and short-lived.
- Pain at the injection site
- Swelling at the injection site
- Fever or light flu-like symptoms
- Fatigue and malaise
In rare cases, patients may experience more serious reactions, such as an allergic reaction or other complications. It is important for individuals to monitor their health after receiving the vaccine and report any unusual symptoms to a healthcare provider promptly. Understanding these risks empowers patients to weigh the benefits of vaccination against possible side effects.
Empowering Patients with Information
In today’s healthcare landscape, empowering patients with information is crucial, especially when it comes to the RSV vaccine. Knowledge allows individuals to make informed decisions regarding their health and wellbeing. When patients understand the details surrounding the RSV vaccine, including its purpose, benefits, and potential risks, they can engage in meaningful discussions with their healthcare providers.
Providing comprehensive information involves several key components. First, patients should be made aware of the transmission and symptoms of Respiratory Syncytial Virus (RSV). Moreover, information about how the vaccine works, its effectiveness, and the importance of vaccination in preventing severe respiratory infections in vulnerable populations is essential. By delivering clear and straightforward details, healthcare professionals enable patients to grasp the significance of the RSV vaccine.
Moreover, patients should feel empowered to ask questions and express concerns about the vaccine. They should also be informed about their rights regarding informed consent. This includes understanding the components of the consent form and being able to identify and evaluate any potential risks and side effects of the vaccination. Such empowerment not only enhances the patient experience but also fosters greater trust between patients and healthcare providers.
Frequently Asked Questions
What is the purpose of the RSV vaccine consent form?
The RSV vaccine consent form is designed to inform patients and guardians about the vaccine, its benefits, potential risks, and to obtain formal consent for its administration.
Who needs to fill out the RSV vaccine consent form?
The RSV vaccine consent form should be filled out by the parent or guardian of the child receiving the vaccine, as well as by any adult who is eligible for the vaccine.
What information is typically included in the RSV vaccine consent form?
The consent form usually includes sections on patient identification, information about the RSV vaccine, a description of potential side effects, and a statement of consent.
Can the RSV vaccine be administered without a consent form?
Generally, the RSV vaccine should not be administered without a completed consent form, as it is crucial to ensure that informed consent has been obtained.
What should individuals do if they have questions about the RSV vaccine before signing the consent form?
Individuals should speak with their healthcare provider to get answers to any questions or concerns they may have about the RSV vaccine prior to signing the consent form.
Is there a specific age group that the RSV vaccine is recommended for?
The RSV vaccine is typically recommended for infants and young children, particularly those at high risk for RSV infection, such as premature infants or those with certain health conditions.
What happens if the consent form is not signed?
If the consent form is not signed, the healthcare provider cannot administer the RSV vaccine, as legal and ethical guidelines require informed consent for vaccination.