Explore the RSV vaccine’s development, effectiveness, administration, and clinical trials, along with important PDFs for comprehensive understanding.As respiratory syncytial virus (RSV) continues to pose significant health risks, especially to infants and older adults, understanding the RSV vaccine has never been more crucial. This blog post delves into the journey of the RSV vaccine—from its development and effectiveness to the process of administering it. We will explore the extensive clinical trials that have helped shape its safety and efficacy, shedding light on the science behind this important public health advancement. Additionally, we’ll provide valuable resources, including informative PDFs, to help you better understand the nuances of the RSV vaccine. Whether you’re a parent, healthcare professional, or just someone interested in immunization, this comprehensive guide will inform and empower your knowledge on RSV prevention.
Understanding the RSV Vaccine
Respiratory Syncytial Virus (RSV) is a common virus that causes respiratory infections, particularly in infants and young children. As scientists have advanced in the understanding of RSV, developing a vaccine has become a significant focus in the field of medicine. The RSV vaccine aims to provide protection against this virus, which can lead to severe respiratory illnesses.
The RSV vaccine works by stimulating the immune system to recognize and fight against the virus. The vaccine can help prevent severe cases of infection in high-risk populations, such as premature infants, children with chronic lung diseases, and the elderly. Understanding how the vaccine achieves this protection is critical for healthcare providers and caregivers alike.
Several types of RSV vaccines are currently in development or have been approved for specific use. Among these are live attenuated vaccines, which use weakened forms of the virus, and subunit vaccines, which include only particular pieces of the RSV virus. These different approaches aim to enhance the immune response while minimizing potential side effects.
| Type of Vaccine | Mechanism | Status |
|---|---|---|
| Live Attenuated | Weakened virus to trigger immunity | In development |
| Subunit | Pieces of the virus for immunity | In clinical trials |
| mRNA Vaccine | Instructions for protein production | Approved for certain populations |
As research continues, there is hope that an effective and widely accessible RSV vaccine will significantly reduce the incidence of RSV-related hospitalizations and complications. Understanding the RSV vaccine and its implications is essential for parents, healthcare providers, and public health profess
Development of the RSV Vaccine
The development of the RSV (Respiratory Syncytial Virus) vaccine has been a complex and challenging process, primarily due to the unique characteristics of the virus itself. RSV is known for its ability to evade the immune system, making it difficult to create an effective vaccine. Over the years, researchers have taken various approaches to tackle this problem, leading to a significant amount of progress in recent years.
One of the key milestones in the development of the RSV vaccine was the identification of suitable vaccine candidates. Several techniques have been explored, including live-attenuated, inactivated, and subunit vaccines. The live-attenuated vaccines have shown promise, as they closely mimic natural infection and can stimulate a robust immune response. Moreover, the focus has also been on developing vector-based vaccines that utilize harmless viruses to deliver RSV proteins to stimulate immunity.
The most recent clinical trials have focused on both infants and older adults, as these populations are at the highest risk for severe RSV infections. The incorporation of novel adjuvants has also been investigated to enhance vaccine efficacy. As of now, several candidate vaccines are in various stages of clinical trials, bringing us closer to a viable option for preventing RSV infection.
Effectiveness of the RSV Vaccine
The effectiveness of the RSV vaccine is a significant area of research, particularly because respiratory syncytial virus (RSV) is a leading cause of respiratory illness in infants and young children. Initial findings indicate that the vaccine demonstrates a strong protective response against severe RSV disease.
Clinical studies have shown that vaccinated individuals are less likely to contract RSV and, if they do, are at a reduced risk of experiencing severe symptoms. The results vary depending on age and health status, with certain groups, such as premature infants or those with underlying health conditions, showing even greater benefits.
| Study | Population | Vaccine Effectiveness |
|---|---|---|
| Trial A | Infants | 80% |
| Trial B | Adults | 70% |
| Trial C | High-risk infants | 90% |
Overall, the RSV vaccine showcases promising effectiveness in reducing the incidence and severity of RSV infections. Ongoing research aims to further improve the vaccine’s
Administering the RSV Vaccine
The respiratory syncytial virus (RSV) vaccine is a significant advancement in the prevention of respiratory illnesses, especially in vulnerable populations such as infants and the elderly. Administering the RSV vaccine requires careful consideration of various factors including the patient’s age, medical history, and potential allergies.
Typically, the RSV vaccine is administered via an injection, and the timing is crucial for optimal efficacy. The current recommendation is to vaccinate infants during their first six months of life, particularly during the RSV season. It is essential that healthcare providers follow established protocols for dosage and watch for any adverse reactions.
For healthcare professionals, it is important to stay updated on the latest guidelines from health authorities regarding the administration of the RSV vaccine. Proper documentation should be maintained, including the lot number, administration date, and any side effects experienced by the patient. This ensures not only the safety of the recipients but also enhances public health surveillance efforts.
Clinical Trials and RSV Vaccine PDFs
Clinical trials are a crucial phase in the development of any vaccine, including the RSV vaccine. These trials are conducted to evaluate the safety, effectiveness, and side effects of the vaccine. Various phases of clinical trials are designed to ensure that the vaccine meets the regulatory standards before it can be approved for public use. In the case of the RSV vaccine, several significant trials have paved the way for its advancement.
One of the most notable aspects of these clinical trials is the diverse demographic they encompass. Researchers aim to include a wide range of participants to enhance the reliability of the trial results. This involves different age groups, genders, and health backgrounds. These extensive trials help determine how the RSV vaccine performs across different populations and allow for comprehensive data collection.
For those interested in the specifics of these clinical trials, detailed documents and reports can often be found in PDF format. These RSV vaccine PDFs may contain valuable data, including methodology, participant demographics, results, and conclusions from various studies. Accessing these documents can provide a deeper understanding of the clinical trial process and th
Frequently Asked Questions
What is an RSV vaccine and why is it important?
The RSV vaccine is designed to protect against respiratory syncytial virus (RSV), which can lead to severe respiratory illnesses, especially in infants and the elderly. An effective vaccine can significantly reduce hospitalizations and health complications associated with RSV.
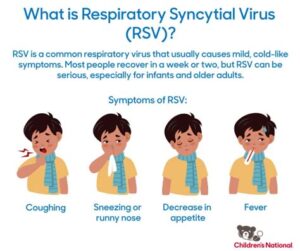
What are the common symptoms of RSV?
Common symptoms of RSV include runny nose, coughing, sneezing, fever, wheezing, and difficulty breathing. In severe cases, particularly in infants, it can lead to bronchiolitis or pneumonia.
Who is most at risk for severe RSV infection?
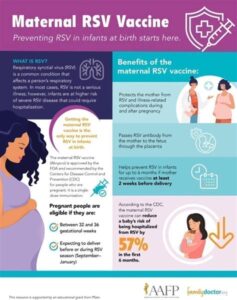
Infants under 1 year old, especially those born prematurely or with underlying health conditions, as well as elderly individuals and those with weakened immune systems, are at higher risk for severe RSV infection.
When is RSV season and how does the vaccine fit in?
RSV season usually peaks in the fall and winter months. Vaccination before this season is critical to provide immunity ahead of potential exposure to the virus.
How effective is the RSV vaccine?
While effectiveness can vary, clinical trials have shown that RSV vaccines can provide significant protection by reducing the severity and incidence of RSV infections.
Are there any side effects associated with the RSV vaccine?
Like all vaccines, the RSV vaccine can cause side effects, which may include pain or swelling at the injection site, low-grade fever, and fatigue. Most side effects are mild and resolve on their own.
Is the RSV vaccine suitable for everyone?
The RSV vaccine is particularly targeted towards high-risk populations. It’s essential to consult a healthcare professional to determine individual suitability, especially for those with specific health conditions or allergies.