Explore the RSV vaccine procedure, its importance, coding guidelines, and documentation requirements to ensure effective and compliant vaccination strategies.As the medical community continues to prioritize the prevention of respiratory illnesses, the respiratory syncytial virus (RSV) vaccine is gaining significant attention. This blog post delves into the essential aspects surrounding RSV vaccination, from understanding the vaccine’s procedure to its vital role in public health. We will explore the procedure code associated with the RSV vaccine, which is crucial for healthcare providers in billing and documentation processes. Additionally, we’ll highlight coding guidelines to ensure accurate claims and reimbursement. Lastly, we will discuss the important documentation requirements that healthcare professionals must adhere to. By the end of this post, you’ll have a comprehensive understanding of RSV vaccine procedures, their importance, and how to navigate the coding landscape effectively.
Understanding RSV Vaccine Procedure
The Respiratory Syncytial Virus (RSV) vaccine is an important advancement in the field of preventive healthcare, particularly for infants and young children who are at the highest risk for severe RSV infections. Understanding the RSV vaccine procedure is crucial for healthcare providers and parents alike to ensure the vaccine is administered correctly and effectively. Below are the key aspects of the RSV vaccine procedure that should be considered.
| Step | Description |
|---|---|
| 1. Screening | The patient should be screened for any contraindications or previous adverse reactions to vaccines. |
| 2. Consent | Obtaining informed consent from the parent or guardian is essential before proceeding with vaccination. |
| 3. Preparation | The vaccine should be prepared according to the manufacturer’s instructions, ensuring proper dilution and storage. |
| 4. Administration | The RSV vaccine should be administered via intramuscular injection, typically in the thigh for infants. |
| 5. Monitoring | Patients should be monitored for at least 15 minutes post-vaccination for any immediate allergic reactions. |
It’s important to note that the RSV vaccine is often given as part of a broader immunization schedule. Healthcare providers should communicate clearly with parents and guardians about the timing of the vaccine, as well as any follow-up doses that may be required based on public health guidelines.
Additionally, record-keeping is vital for vaccines like the RSV vaccine. This includes documenting the vaccine lot number, administration site, and the date of administration. Such documentation not only helps in tracking the vaccination status of the patient but also aids in compliance with local and federal health regulations.
In conclusion, understanding the RSV vaccine procedure is critical for ensuring the safety and health of vulnerable populations. By following these guidelines, healthcare providers can effectively cont
Importance of RSV Vaccine
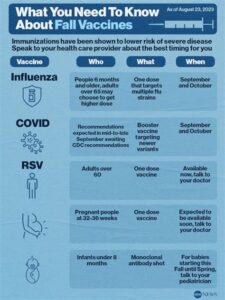
The RSV vaccine is crucial for protecting vulnerable populations, particularly infants and older adults, from Respiratory Syncytial Virus (RSV). RSV is a highly contagious virus that can lead to severe respiratory illnesses, including bronchiolitis and pneumonia. By implementing an RSV vaccination strategy, the healthcare community can significantly reduce the incidence of these severe outcomes.
One of the primary reasons the RSV vaccine is essential is that it helps boost immunity in at-risk populations. Infants, especially those born prematurely or with underlying health conditions, are particularly susceptible to severe RSV infections. Vaccination not only protects these infants but also helps minimize the risk of transmission within communities. With widespread adoption of the vaccine, we can create a herd immunity effect that can safeguard more vulnerable individuals.
Furthermore, the importance of RSV vaccine extends beyond immediate health benefits. Reducing the incidence of severe RSV cases can alleviate the burden on healthcare systems. Hospitalizations due to RSV can strain medical resources, especially during peak seasons. By decreasing the number of infections, we can ensure that healthcare providers are better equipped to manage other medical needs in the community.
Procedure Code for RSV Vaccine
The procedure code for the RSV vaccine is crucial for proper billing and documentation in healthcare settings. Understanding this code ensures that healthcare providers can accurately track immunizations and facilitate reimbursements from insurance companies.
The Current Procedural Terminology (CPT) code for the RSV vaccine, specifically for the administration of palivizumab, is typically 90378. This code is designated for the preventive treatment of RSV in high-risk infants and young children, representing a significant step in reducing respiratory infections.
| Code | Description | Administration Guidelines |
|---|---|---|
| 90378 | RSV vaccine administration for high-risk children | Given monthly during RSV season for eligible infants |
Healthcare providers must ensure they are using the correct procedure code to promote efficient coding practices and maintain records for vaccine administration effectively.
Coding Guidelines for RSV Vaccine
The RSV vaccine is essential in protecting vulnerable populations against respiratory syncytial virus. To ensure proper billing and reimbursement, it is crucial to adhere to the correct coding guidelines associated with the RSV vaccine. This includes the identification of appropriate codes to accurately classify both the vaccine and its administration.
When coding for the RSV vaccine procedure, healthcare providers should be familiar with the specific procedure codes established for the administration of this vaccine.
| Code | Description |
|---|---|
| 90705 | Respiratory Syncytial Virus Vaccine |
| 90460 | Immunization Administration |
| 90461 | Each additional vaccine administered |
In addition to selecting the proper procedure code, it is vital to keep updated on coding guidelines provided by organizations such as the American Academy of Pediatrics (AAP) and the Centers for Medicare & Medicaid Services (CMS). Following these guidelines will facilitate smoother reimbursement processes and reduce the likelihood of claim denials.
Furthermore, documentation is also an integral part of the coding process. Ensure that all details regarding the administration of the RSV vaccine, including patient information, vaccine lot numbers, and administration sites, are meticulously recorded. This documentation supports the coding submitted to insurance
Documentation Requirements for RSV Vaccine
When it comes to the RSV vaccine, proper documentation is crucial for ensuring compliance with medical guidelines, reimbursement processes, and public health tracking. Health care providers must thoroughly document each step of the vaccination process.
- Patient Information: Include the patient’s full name, date of birth, and any identification numbers.
- Date of Administration: Clearly note when the vaccination was administered.
- Vaccine Details: Document the specific RSV vaccine given, including the lot number and expiration date.
- Dosage Information: Record the dosage administered.
- Provider Information: The name and credentials of the healthcare provider who administered the vaccine.
- Side Effects Monitoring: It is essential to monitor and document any immediate side effects post-administration.
Additionally, maintaining a vaccination log is critical for tracking the status of each patient’s immunization against RSV. This ensures that subsequent doses are appropriately scheduled and administered according to medical guidelines.
Lastly, healthcare facilities should also ensure that they comply with any federal or state-specific documentation requirements related to vaccination programs. Keeping accurate and comprehensive records not only aids in patient care but also plays a significant role in broader public health initiatives.
Frequently Asked Questions
What is the purpose of the RSV vaccine?
The RSV vaccine is designed to protect individuals, especially infants and older adults, from respiratory syncytial virus (RSV), which can cause severe respiratory infections.
What procedure codes are used for RSV vaccine administration?
The procedure codes for RSV vaccine administration may include specific CPT codes utilized by healthcare providers to bill for the vaccine, such as 90677 for the monoclonal antibody.
How does the RSV vaccine work?
The RSV vaccine works by stimulating the immune system to recognize and fight off the respiratory syncytial virus, thereby reducing the risk of severe illness.
Who is recommended to receive the RSV vaccine?
The RSV vaccine is generally recommended for infants under 2 years old, particularly those at high risk for severe RSV disease, as well as older adults with significant comorbid conditions.
What are common side effects of the RSV vaccine?
Common side effects of the RSV vaccine may include mild soreness at the injection site, low-grade fever, and fatigue, though severe side effects are rare.
How is the RSV vaccine administered?
The RSV vaccine is typically administered via an intramuscular injection, often given in a pediatric practice or healthcare setting.
What should parents do if they have concerns about the RSV vaccine?
Parents should consult their child’s healthcare provider for personalized advice and information regarding the RSV vaccine, including its benefits and potential risks.