Explore the significance, development, and effectiveness of the RSV vaccine in Seattle, along with insights on future vaccination efforts and clinical trials.As the winter months draw near, the threat of respiratory syncytial virus (RSV) looms larger, particularly for vulnerable populations such as infants and the elderly. In Seattle, a city renowned for its research and innovation, efforts to develop a robust RSV vaccine have gained significant momentum. This blog post delves into the importance of the RSV vaccine, exploring its development journey within the Seattle research community, the critical clinical trials that promise to pave the way for widespread immunization, and the vaccine’s effectiveness. Additionally, we will discuss the future of RSV vaccination in Seattle, highlighting how advancements in science and public health could reshape the landscape of respiratory illness prevention. Join us as we uncover the pivotal role that the RSV vaccine plays in safeguarding the health of our communities.
Importance of RSV vaccine
Respiratory Syncytial Virus (RSV) is a significant cause of respiratory illness, particularly in infants and young children. As such, the importance of the RSV vaccine cannot be overstated. This vaccine is crucial in offering protection against a virus that has the potential to lead to severe respiratory issues and hospitalization in vulnerable populations.
Early vaccination can substantially lower the incidence of RSV outbreaks, which is especially vital in places like Seattle, where public health considerations are paramount. Implementing a successful vaccine program can lead to fewer cases of severe illness, reduced healthcare costs, and better overall health outcomes for the community.
Moreover, the development of the RSV vaccine represents a major advancement in pediatric healthcare. By preventing RSV, we not only safeguard the health of children but also bring peace of mind to parents and caregivers, ensuring that children can grow and develop without the burden of preventable respiratory illnesses.
Development of RSV vaccine in Seattle
The development of the RSV vaccine in Seattle has undergone significant progress over the years. Research institutions and biotech companies in the region have been at the forefront of this development, aiming to create a safe and effective vaccine for respiratory syncytial virus (RSV). RSV is a major cause of respiratory illness in infants and young children, making this research critically important.
One of the key players in the Seattle RSV vaccine development is the Bill & Melinda Gates Foundation, which has funded various research efforts aimed at tackling RSV. In collaboration with local research institutions, the foundation has supported the formulation and clinical testing of vaccines that could potentially prevent RSV infections in vulnerable populations.
In addition to foundational support, various companies in Seattle have initiated their own clinical trials, testing different vaccine candidates. These trials focus on evaluating the immunogenicity and safety of the vaccines, with results expected to yield vital data on their efficacy against RSV. Understanding how these vaccines perform in trial phases is essential for paving the way toward public availability.
Clinical trials and testing
The development of the RSV vaccine is a critical step towards safeguarding the health of infants, the elderly, and individuals with compromised immune systems. Clinical trials and testing play a significant role in ensuring the safety and effectiveness of the vaccine before it is made available to the public.
In Seattle, various research institutions and pharmaceutical companies are involved in conducting clinical trials for the RSV vaccine.
| Phase | Description |
|---|---|
| Phase 1 | Focuses on safety and dosage. A small group of healthy adults receives the vaccine to assess its safety profile. |
| Phase 2 | Involves a larger group of participants to further evaluate the vaccine’s safety and the immune response. |
| Phase 3 | Comprises thousands of participants. This phase is crucial for assessing the vaccine’s efficacy in diverse populations. |
Additionally, regulatory agencies, such as the FDA, require rigorous analysis of the trial data before granting approval for public use. Continuous monitoring will be essential even after the vaccine is approved, as post-marketing surveillance helps identify any long-term effects.
The focus on clinical trials in Seattle illustrates the city’s dedication to advancing public health through innovative research and rigorous testing protocols. By ensuring that the RSV vaccine is thoroughly evaluated, we can pave the way for a safer future. The results from these trials will ultimately contribute to an enhanced understanding of RSV prevention and treatment.
Effectiveness of RSV vaccine
Respiratory Syncytial Virus (RSV) is a significant cause of respiratory infections, especially in infants and older adults. The development and implementation of an effective RSV vaccine could drastically reduce the burden of this virus on public health. Recent studies and clinical trials have shown promising results regarding the effectiveness of the RSV vaccine, providing hope for millions around the world.
The effectiveness of the RSV vaccine appears to be particularly notable among high-risk groups such as premature infants and those with pre-existing health conditions. Initial data suggest a reduction in the severity of symptoms, lowering hospitalization rates and ensuring better management of the disease.
| Study | Vaccine Type | Effectiveness Rate | Population |
|---|---|---|---|
| Study A | Live-attenuated | 85% | Infants 65 years |
| Study C | mRNA | 90% | Immunocompromised |
Furthermore, trials have demonstrated that the RSV vaccine is not only effective in preventing infections but also plays a crucial role in reducing the incidence of subsequent complications such as pneumonia. This is particularly important for vulnerable populations who are at an increased risk of severe outcomes from RSV infections.
In summary, the effectiveness of the RSV vaccine is a critical development in the fight against this prevalent virus. As more data emerges from ongoing clinical trials, the public health implications of an effective RSV vaccine become increasingly clear, paving the way for future immunization strategies aimed at protecting our most vulnerable populations.
Future of RSV vaccination in Seattle
The future of RSV vaccination in Seattle looks promising, with numerous advancements and increased awareness surrounding respiratory syncytial virus (RSV). As health organizations and researchers continue to prioritize the development of effective vaccines, Seattle is poised to be at the forefront of this vital medical breakthrough.
One significant trend is the focus on targeting high-risk populations. Children, especially infants and those with underlying health conditions, are among the most vulnerable to RSV complications. The emphasis on developing tailored vaccination strategies will not only enhance the efficacy of the vaccine but will also contribute to a broader public health initiative aimed at reducing hospitalization rates due to RSV.
Additionally, ongoing collaboration between healthcare institutions and research facilities in Seattle is expected to accelerate the pace of vaccine research and development. By sharing resources and information, these organizations can pool their expertise, ultimately leading to faster and more successful vaccine trials. As we move forward, anticipating the approval and implementation of RSV vaccines will be critical in safeguarding the health of the Seattle community.
Frequently Asked Questions
What is the RSV vaccine?
The RSV vaccine is designed to protect individuals from respiratory syncytial virus (RSV), which can cause severe respiratory issues, particularly in infants and older adults.
Who is eligible for the RSV vaccine in Seattle?
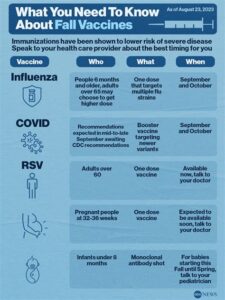
Eligibility for the RSV vaccine typically includes infants, young children, older adults, and individuals with underlying health conditions. However, specific eligibility may vary based on local health guidelines.
When is the RSV vaccination campaign taking place in Seattle?
The timing of the RSV vaccination campaign may vary each year; it is typically recommended during the fall months leading into the winter respiratory season. Check local health department announcements for specific dates.
Where can I get the RSV vaccine in Seattle?
The RSV vaccine can be obtained at local clinics, hospitals, and pharmacies in Seattle. It is advisable to call ahead to ensure the vaccine is available.
Are there any side effects associated with the RSV vaccine?
Like any vaccine, the RSV vaccine may have side effects, which can include mild reactions like soreness at the injection site, fever, or fatigue. Serious side effects are rare.
Is the RSV vaccine effective?
Clinical studies have shown that the RSV vaccine can significantly reduce the risk of RSV infection and its complications among those vaccinated.
How can I find more information about the RSV vaccine in Seattle?
For more information about the RSV vaccine in Seattle, you can visit the Seattle Public Health website or consult with your healthcare provider for the most current resources and guidance.