Explore the benefits, effectiveness, and future prospects of combining RSV and flu vaccines, supported by clinical trials and research insights.As we continue to navigate the challenges of respiratory illnesses, the discussion around vaccinations becomes increasingly vital. Among the most noteworthy developments are the RSV (Respiratory Syncytial Virus) vaccine and the flu vaccine, both pivotal in preventing serious respiratory infections, particularly in vulnerable populations. In this blog post, we will explore the implications of combining these vaccines, highlighting the potential benefits and the ongoing clinical trials investigating their interaction. We will also delve into the effectiveness of administering both vaccines simultaneously, shedding light on the promising future of respiratory virus research. With respiratory infections remaining a significant public health concern, understanding these vaccines’ synergies could play a crucial role in enhancing community immunity and safeguarding health. Join us as we unpack this important topic.
Understanding RSV vaccine and flu vaccine
Respiratory Syncytial Virus (RSV) and influenza (flu) are both significant viral infections that can lead to severe respiratory illness, particularly in vulnerable populations such as infants, the elderly, and those with pre-existing health conditions. Understanding the nuances of the RSV vaccine and the flu vaccine is essential in mitigating the impact of these viruses on public health.
The RSV vaccine is designed to provide immunity against RSV infections, which are one of the leading causes of acute respiratory illness in children under two years old. The vaccine works by stimulating the immune system to recognize and fight the RSV virus effectively. On the other hand, the flu vaccine aims to protect against the seasonal influenza virus, which changes every year. Due to this variability, annual vaccination is necessary to ensure optimal protection.
Both vaccines play a crucial role in health programs globally, particularly in preventing hospitalization and reducing mortality rates associated with these viral infections. Furthermore, understanding the differences and similarities in their formulation and administration can help health professionals advocate effectively for their use as part of a comprehensive public health strategy.
Benefits of combining RSV and flu vaccines
The potential benefits of combining the RSV (Respiratory Syncytial Virus) and flu (influenza) vaccines are becoming increasingly noteworthy in the field of immunology. As both viruses primarily affect the respiratory system, co-administering these vaccines can significantly enhance patient protection, particularly in vulnerable populations such as infants, the elderly, and individuals with pre-existing health conditions.
One of the prominent advantages of this combination is the convenience it offers. Patients can receive protection from two significant respiratory illnesses in a single visit, reducing the burden of multiple appointments and improving vaccination rates. This convenience can lead to increased community immunity and contribute to the mitigation of seasonal outbreaks.
Additionally, early research suggests that the immune response generated by combining both vaccines could be synergistic, potentially leading to a stronger defense against both RSV and influenza. This synergy could not only enhance vaccine efficacy but also simplify public health strategies by streamlining vaccination efforts during peak respiratory illness seasons.
Clinical trials on RSV and flu vaccine interaction
Recent advances in vaccine development have sparked significant interest in the interaction between the RSV vaccine and the flu vaccine. Researchers are conducting numerous clinical trials to evaluate how these vaccines can be co-administered and whether their combined efficacy can lead to enhanced protection against respiratory infections.
One of the primary goals of these trials is to establish the safety and effectiveness of administering the RSV and flu vaccines simultaneously. Preliminary results from various studies indicate that co-administration does not compromise the immune response to either virus. In fact, it may provide synergistic effects, offering broader protection, especially in vulnerable populations such as infants, the elderly, and those with underlying health conditions.
Moreover, clinical trials are focusing on assessing the immunogenicity of the combined vaccines. Researchers have been documenting biomarkers and titers in participants to understand how the body responds when exposed to both vaccines at the same time. This data will be crucial for formulating guidelines for integrated vaccination strategies in the future.
“These clinical trials are essential to paving the way for more efficient vaccination practices that could save lives and reduce the burden of respiratory illnesses globally.”
| Trial Phase | Participants | Start Date | Status |
|---|---|---|---|
| I | 100 | Jan 2023 | Completed |
| II | 500 | Mar 2023 | Ongoing |
| III | 1500 | Expected May 2024 | Pending |
Effectiveness of co-administering RSV and flu vaccines
The co-administration of the RSV (Respiratory Syncytial Virus) vaccine and the influenza (flu) vaccine has been a subject of increasing interest among healthcare professionals. This is particularly relevant in the context of public health, especially during the respiratory virus seasons. Research indicates that combining these two vaccines may enhance overall protective immunity against respiratory viruses, which are particularly harmful in vulnerable populations such as children and the elderly.
One of the primary benefits of co-administration is the convenience it offers. Patients can receive protection from both RSV and flu during a single visit, reducing the need for multiple appointments. This is especially beneficial for at-risk populations who may have difficulty accessing healthcare services frequently. Furthermore, studies have suggested that the safety profile of administering both vaccines concurrently is comparable to administering them separately, which is a positive finding for vaccine administration practices.
Clinical data collected from trials indicates that the immune response against both viruses is adequately generated, and there have been no significant adverse effects reported from the combination. As the public health landscape evolves, understanding the effectiveness of co-administering vaccines becomes crucial in optimizing imm
Future prospects for RSV and flu vaccine research
The future of RSV and flu vaccine research holds immense potential, particularly as public health demands evolve. Researchers are exploring innovative approaches to enhance vaccine effectiveness and broaden coverage among various age groups. One of the most promising directions involves integrating both RSV and flu vaccines into a single formulation, potentially streamlining immunization processes.
Advancements in mRNA technology, previously utilized in COVID-19 vaccines, could also pave the way for more effective RSV and flu vaccines. By employing this rapid development technique, scientists might be able to create vaccines that provide robust protection with fewer side effects. Additionally, studies are being conducted on the longevity of immunity provided by current vaccines, and this information will be critical in determining future vaccination strategies.
Moreover, understanding the interactions between these vaccines is vital for ensuring safety and maximizing efficacy. Ongoing clinical trials are shedding light on the co-administration of RSV and flu vaccines, which could lead to combination vaccines that protect against multiple respiratory viruses in one shot. These developments could significantly improve vaccination rates, reduce disease burden, and ultimately enhance public health.
Frequently Asked Questions
What is the purpose of combining the RSV vaccine with the flu vaccine?
Combining the RSV vaccine with the flu vaccine aims to streamline vaccination efforts, improve public health outcomes, and enhance the immune response against both respiratory syncytial virus (RSV) and influenza.
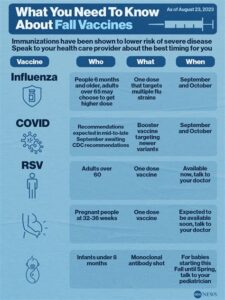
Who is recommended to receive the RSV and flu vaccines?
The RSV vaccine is typically recommended for high-risk groups, such as infants and elderly individuals, while the flu vaccine is advised for almost everyone over the age of 6 months.
What are the potential benefits of receiving both vaccines together?
Receiving both vaccines together can reduce the likelihood of developing serious respiratory infections, decrease healthcare costs, and improve overall community immunity.
Are there any known side effects of getting both vaccines at the same time?
Common side effects may include mild soreness at the injection site, fatigue, and low-grade fever, but these are generally short-lived and less severe than the illnesses they prevent.
How do RSV and flu viruses compare in terms of severity?
While both RSV and influenza can cause severe respiratory illness, RSV is a leading cause of hospitalization in infants and young children, whereas influenza can affect individuals of all ages, particularly those with compromised health.
Is the RSV vaccine available for adults?
As of now, the RSV vaccine is primarily available for infants, with ongoing research and development for vaccines targeted specifically at older adults.
What is the recommended vaccination schedule for RSV and flu vaccines?
The flu vaccine is recommended annually, while the RSV vaccine schedule may vary depending on the specific vaccine approved for use, so it’s important to follow healthcare provider guidelines.