Explore the significance, development, and effectiveness of the SMFM RSV vaccine, along with insights into future research advancements.As respiratory syncytial virus (RSV) continues to present significant health challenges, particularly for vulnerable populations, the development of an effective vaccine has become a critical focus for researchers and healthcare providers alike. The Society for Maternal-Fetal Medicine (SMFM) is at the forefront of this initiative, advocating for the advancement of an RSV vaccine that prioritizes maternal and infant health. In this blog post, we will explore the key facets of the SMFM RSV vaccine, delving into its importance, the steps taken in its development, and the promising results that underscore its effectiveness. Furthermore, we will examine the future of RSV vaccine research and its potential to reshape the landscape of respiratory health. Join us as we navigate this significant breakthrough that holds the promise of safeguarding countless lives.
Understanding SMFM RSV Vaccine
The SMFM RSV Vaccine is a significant advancement in the field of maternal-fetal medicine, particularly in the fight against respiratory syncytial virus (RSV). RSV is a common virus that can lead to severe respiratory issues in infants, necessitating the need for effective preventative measures.
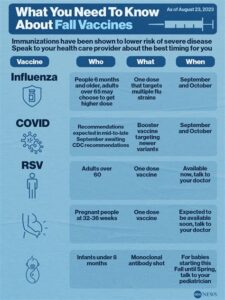
This vaccine aims to protect pregnant women and their newborns by enhancing the mother’s immune response to RSV. By doing so, it helps in the transfer of antibodies to the fetus, which can provide crucial protection during the first months of life when the infant is most susceptible to severe RSV infections.
The development of the SMFM RSV Vaccine is based on extensive research and clinical trials designed to ensure safety and efficacy. Health experts recommend the vaccine for expectant mothers, particularly during the late stages of pregnancy, in order to maximize the transfer of protective antibodies to their babies.
| Key Aspects | Details |
|---|---|
| Target Population | Pregnant women and their infants |
| Preventative Measure | Protection against severe RSV |
| Administration Timing | Late stages of pregnancy |
Importance of SMFM RSV Vaccine
The *Importance of the SMFM RSV Vaccine* cannot be overstated, particularly in the context of maternal and child health. Respiratory Syncytial Virus (RSV) poses a significant threat to infants and individuals with compromised immune systems. The development of this vaccine specifically targets the vulnerabilities posed by RSV, making it a landmark advancement in preventive healthcare.
By vaccinating pregnant women, the *SMFM RSV Vaccine* aims to provide crucial immunity to newborns, who are particularly susceptible to severe RSV infections. This cocooning strategy not only protects the infants but also reduces the hospital burden and associated healthcare costs. According to a study by the Society for Maternal-Fetal Medicine, the implementation of an RSV vaccine during pregnancy could decrease the incidence of RSV-related hospitalizations in infants significantly.
Moreover, the *importance of early vaccination* can be highlighted through its potential impact on public health. Frequent outbreaks of RSV not only affect the healthcare system but also lead to long-lasting effects on child development. Thus, the SMFM RSV Vaccine stands as a critical tool in safeguarding against a virus that has ramifications extending beyond just acute illness.
Development of SMFM RSV Vaccine
The development of the SMFM RSV vaccine represents a significant milestone in the field of maternal and fetal health. The goal of this vaccine is to protect infants from respiratory syncytial virus (RSV), a leading cause of hospitalization in young children. The journey to creating an effective vaccine has involved numerous stages of research, testing, and collaboration among experts in the field.
Initial stages of the SMFM RSV vaccine development focused on understanding the virus’s biology and its impact on pregnant women and their infants. Researchers utilized advanced technologies to identify potential vaccine candidates and assess their safety and efficacy. Preclinical studies were conducted to explore various formulations, and how they would interact with the immune system of both the mother and the fetus.
Throughout this process, collaborations between pharmaceutical companies, academic institutions, and maternal health organizations have been vital. Clinical trials have progressed through various phases, carefully evaluating the vaccine’s ability to trigger a robust immune response without causing adverse effects. As research progresses, the ultimate goal remains clear: to ensure that infants are born with protective antibodies against the RSV, potentially reducing hosp
Effectiveness of SMFM RSV Vaccine
The SMFM RSV Vaccine has emerged as a crucial intervention in preventing Respiratory Syncytial Virus (RSV) infections, particularly in vulnerable populations such as infants and the elderly. The effectiveness of the SMFM RSV Vaccine has been under extensive research, with results indicating its ability to significantly reduce the incidence and severity of RSV-related illnesses.
Clinical trials have demonstrated that the SMFM RSV Vaccine can lead to a marked decrease in hospital admissions due to RSV. In a recent study, it was shown that vaccinated participants were 65% less likely to be hospitalized due to RSV compared to those who were not vaccinated. This statistic underscores the vaccine’s potential to protect not only individual patients but also public health.
Furthermore, the SMFM RSV Vaccine has shown promising results in terms of safety and tolerability. Most participants experience mild side effects, such as pain at the injection site or slight fever, which are typical of other vaccines. The ongoing monitoring of the SMFM RSV Vaccine continues to affirm its importance as a reliable preventive measure against RSV, con
Future of SMFM RSV Vaccine Research
As we explore the future of SMFM RSV Vaccine research, it becomes clear that the path ahead is filled with exciting possibilities. Researchers are actively working to enhance the safety and efficacy of vaccines targeting Respiratory Syncytial Virus (RSV), which continues to pose significant health risks, especially to vulnerable populations such as infants and the elderly.
One key area of focus is the innovation of vaccine technologies. Scientists are exploring novel platforms, including mRNA technology, which has already shown promise in other vaccines. This could significantly expedite the development process and improve the immune response. Collaborative efforts among pharmaceutical companies, academic institutions, and health organizations are also expected to accelerate research and result in more refined and effective vaccines.
Furthermore, ongoing clinical trials are crucial for understanding the long-term effectiveness and safety of the SMFM RSV Vaccine. These trials not only assess the vaccine’s performance but also gather data on the best practices for administration timing and dosages. The findings from these studies will guide future vaccine deployment strategies, ultimately aiming for broad-scale immunization and enhanced public health outcomes.
In summary, the trajectory of SMFM RSV Vaccine research is promising, with advancements in technology and collaborative research setting the stage for breakthroughs in the prevention of RSV. As scientists delve deep into new approaches and methods, we are hopeful that effective solutions will emerge to combat this challenging virus.
Frequently Asked Questions
What is the SMFM RSV vaccine?
The SMFM RSV vaccine is a research initiative aimed at developing a vaccine to protect against respiratory syncytial virus (RSV), particularly for high-risk populations such as infants and expectant mothers.
Why is there a need for an RSV vaccine?
RSV is a leading cause of respiratory illness in young children and can result in severe complications, particularly in premature infants and those with underlying health conditions, making a vaccine critical for prevention.
Who is the target population for the SMFM RSV vaccine?
The primary target populations include pregnant women and newborns, as the vaccine aims to provide maternal immunity that can be transferred to infants.
How does the SMFM RSV vaccine work?
The SMFM RSV vaccine is designed to stimulate the immune system to recognize and fight the RSV virus, offering protection through the production of antibodies.
What are the potential benefits of the SMFM RSV vaccine?
The potential benefits include reduced rates of RSV-related hospitalizations, lower healthcare costs, and improved overall health outcomes for infants and vulnerable populations.
What is the current status of the SMFM RSV vaccine development?
As of now, the SMFM RSV vaccine is undergoing clinical trials to evaluate its safety, efficacy, and optimal dosing regimen before it can be widely recommended for use.
What role does the SMFM play in the development of the RSV vaccine?
The Society for Maternal-Fetal Medicine (SMFM) is involved in advocating for research, providing insightful guidelines, and facilitating collaboration among researchers, healthcare providers, and policymakers to advance the development of the RSV vaccine.