Discover the Wegmans RSV vaccine, its development, clinical trials, effectiveness, and availability in this comprehensive guide. Stay informed about this crucial vaccine!As the focus on respiratory health continues to rise, the Wegmans RSV vaccine emerges as a promising advancement in combating respiratory syncytial virus (RSV) infections, particularly in vulnerable populations like infants and the elderly. This blog post will explore the key aspects of the Wegmans RSV vaccine, starting with an overview of what it is and its significance in public health. We will delve into the vaccine’s development journey, discuss the clinical trials that validate its effectiveness, and outline its anticipated availability and distribution. By understanding these facets, we can better appreciate how the Wegmans RSV vaccine stands to make a substantial impact on safeguarding respiratory health in our communities. Join us as we unpack the narrative behind this innovative vaccine and its potential to change lives.
What is the Wegmans RSV vaccine?
The Wegmans RSV vaccine is a groundbreaking immunization designed to protect individuals from respiratory syncytial virus (RSV), a common virus that can lead to serious respiratory issues, particularly in infants, elderly individuals, and those with weakened immune systems. This vaccine represents a significant advancement in the fight against RSV, offering hope for more effective prevention strategies in vulnerable populations.
Developed by Wegmans Pharmaceuticals, the RSV vaccine employs innovative technology to stimulate the immune system, enhancing the body’s natural defense mechanisms against the virus. Clinical data suggests that the vaccine can effectively reduce the incidence of RSV-related hospitalizations and severe respiratory complications, making it a vital tool for public health.
In addition to protecting at-risk populations, the Wegmans RSV vaccine holds promise for broader applications and may ultimately contribute to lowering the overall burden of respiratory illnesses associated with RSV in the community. It illustrates the ongoing commitment to advancing vaccine technologies and improving health outcomes for individuals of all ages.
Development of the Wegmans RSV vaccine
The Wegmans RSV vaccine has been a significant focal point in the fight against respiratory syncytial virus (RSV), a condition that primarily affects infants and the elderly. The vaccine development process has undergone several stages, from initial research and discovery to clinical trials. The journey began with understanding the virus’s structure and identifying potential antigens that could elicit a strong immune response.
Initially, researchers collaborated with virologists and immunologists to identify the key components of the RSV that could be used to formulate the vaccine. This involved analyzing the virus’s genome and utilizing advanced technology to pinpoint effective vaccine candidates. The goal was to develop a vaccine that provided robust immunity while being safe for populations at risk, especially infants.
As the research progressed, the Wegmans team transitioned to preclinical testing, where they evaluated the safety and efficacy of their vaccine candidates in laboratory settings and animal models. Positive results from these experiments enabled the team to move forward into clinical trial phases, which are crucial for determining the vaccine’s effectiveness and potential side effects in human subjects.
Clinical trials for the Wegmans RSV vaccine
The clinical trials for the Wegmans RSV vaccine have been conducted to ensure its safety and effectiveness. These trials have followed rigorous protocols and standards set by health authorities. The trials are divided into several phases, each designed to gather important data on how the vaccine performs.
During the Phase 1 trials, the focus was primarily on safety and determining the appropriate dosage. A small group of volunteers received the vaccine, allowing researchers to monitor any adverse effects. This phase was critical in establishing that the vaccine could be administered without severe side effects.
Next came the Phase 2 trials, which involved a larger cohort of participants to evaluate the immunogenicity of the vaccine. This phase aimed to understand how well the body responds to the vaccine and whether it produces sufficient antibodies against the Respiratory Syncytial Virus (RSV). The promising results from Phase 2 led to the initiation of Phase 3 trials.
In the ongoing Phase 3 trials, the Wegmans RSV vaccine is being tested in a much larger population. This phase aims to measure the vaccine’s effectiveness in preventing RSV infections in various demographic groups, including infants and older adults. Preliminary results indicate a strong potential for reducing RSV-related hospitalizations.
| Phase | Description | Focus |
|---|---|---|
| Phase 1 | Safety and dosage | Monitor adverse effects |
| Phase 2 | Immunogenicity | Antibody response |
| Phase 3 | Effectiveness | Prevent RSV infections |
The results from these clinical trials will be crucial for determining the approval and wider availability of the Wegmans RSV vaccine. Ongoing monitoring and further studies will likely continue even after the vaccine receives regulatory clearance to ensure its long-term effectiveness and safety.
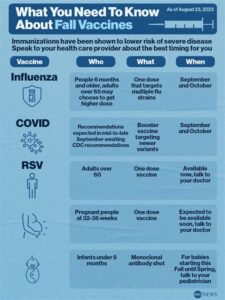
Effectiveness of the Wegmans RSV vaccine
The Wegmans RSV vaccine has generated significant interest in the medical community due to its potential effectiveness in combating respiratory syncytial virus (RSV). This virus is a common cause of respiratory infections, particularly in infants and the elderly, leading to severe health risks. Clinical research has aimed to determine how well this vaccine can protect against RSV infection.
In clinical trials, the effectiveness of the Wegmans RSV vaccine has shown promising results. For instance, data indicates a significant reduction in the incidence of RSV-related hospitalizations among vaccinated individuals compared to those who received a placebo. This statistic highlights the vaccine’s potential to not only prevent infection but also reduce severe disease outcomes associated with RSV.
Moreover, the vaccine exhibits a favorable safety profile, with most side effects being mild and transient. As public interest grows, ongoing studies will continue to evaluate the long-term effectiveness and safety of the Wegmans RSV vaccine, aiming to establish its role as a crucial tool in managing RSV in vulnerable populations.
Availability and distribution of the Wegmans RSV vaccine
The Wegmans RSV vaccine has generated considerable interest in public health circles due to its potential to mitigate the impact of respiratory syncytial virus (RSV), especially in vulnerable populations such as infants and the elderly. The distribution of this vaccine is crucial to ensure that it reaches those who need it the most.
Currently, distribution efforts are focused on a phased rollout strategy. This method allows for targeted delivery to high-risk groups initially, ensuring that those most vulnerable to RSV are vaccinated first. As production capacity increases, the vaccine will become more broadly available to the general public.
Moreover, partnerships with healthcare providers, pharmacies, and community health organizations will play a vital role in the availability of the Wegmans RSV vaccine. Public health campaigns are also expected to raise awareness and educate the community about the benefits of vaccination against RSV.
Frequently Asked Questions
What is the Wegmans RSV vaccine?
The Wegmans RSV vaccine is a vaccine developed to provide protection against Respiratory Syncytial Virus (RSV), which is a common respiratory virus that can cause serious illness in infants and older adults.
Who should consider getting the Wegmans RSV vaccine?
The Wegmans RSV vaccine is primarily recommended for infants, young children, older adults, and individuals with weakened immune systems or underlying health conditions.
How does the Wegmans RSV vaccine work?
The vaccine works by stimulating the immune system to recognize and fight the RSV virus, thereby reducing the risk of severe illness and complications associated with the virus.
What are the side effects of the Wegmans RSV vaccine?
Common side effects may include mild pain at the injection site, fatigue, headache, and fever. Serious side effects are rare but should be discussed with a healthcare provider.
When is the best time to get the Wegmans RSV vaccine?
It is best to get the vaccine before RSV season, which typically runs from fall through spring, to ensure adequate protection during peak times.
Is the Wegmans RSV vaccine safe?
Yes, the Wegmans RSV vaccine has undergone rigorous testing to ensure its safety and efficacy. However, individuals should consult their healthcare provider to discuss any specific concerns.
Where can I get the Wegmans RSV vaccine?
The Wegmans RSV vaccine is available at participating Wegmans pharmacies and healthcare facilities. It is advisable to check with your local Wegmans for availability and make an appointment.