Explore the latest updates on RSV vaccine development, including Kaiser’s progress, challenges faced, and the potential impact on public health. Stay informed!As respiratory syncytial virus (RSV) continues to pose significant health risks, especially for infants and the elderly, the quest for an effective vaccine has become increasingly paramount. The anticipation surrounding Kaiser Permanente’s efforts in developing an RSV vaccine is palpable, as many eagerly await a breakthrough in this area. In this blog post, we’ll explore the background of the RSV vaccine, examine the current status of Kaiser’s development efforts, and outline the expected timeline for its release. We’ll also delve into the challenges faced in vaccine development and discuss the potential impact of an RSV vaccine on public health. Join us as we navigate this critical topic that could change the landscape of respiratory illness prevention.
Background of RSV Vaccine
Respiratory Syncytial Virus (RSV) is a common virus that causes respiratory infections, particularly in infants and young children. Researchers have been striving to develop a vaccine to combat this virus, as it leads to significant morbidity and mortality among vulnerable populations.
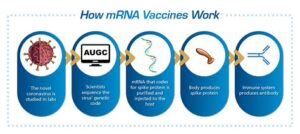
Historically, conventional vaccine strategies faced numerous challenges due to the unique properties of RSV. Initial attempts during the 1960s resulted in enhanced disease in vaccinated children, leading to the abandonment of those early vaccine candidates. However, recent advancements in vaccine technology, including mRNA and viral vector platforms, have reignited interest and optimism regarding RSV vaccine development.
The immune response elicited by RSV infections is complex, and developing a vaccine that can generate a strong and lasting immunity without causing enhanced disease remains a critical goal. As scientists analyze new strategies, the focus has shifted toward creating safer and more effective vaccine options for all populations, particularly the most vulnerable, such as infants, the elderly, and those with compromised immune systems.
Status of Kaiser’s RSV Vaccine Development
The development of the RSV vaccine by Kaiser Permanente has garnered significant attention in recent years. Researchers at Kaiser have been working diligently to create a vaccine that can effectively protect individuals from the respiratory syncytial virus (RSV), especially among vulnerable populations such as infants and the elderly.
As of now, the vaccine development process is progressing through various stages, including preclinical trials and early-phase human trials. Preliminary results have shown promising efficacy, indicating that the vaccine may provide substantial immunity against RSV infection. The focus has been on ensuring the safety and efficacy of the vaccine, which are critical for public acceptance and health recommendations.
Kaiser Permanente is collaborating with other research institutions and industry leaders to accelerate the development of the RSV vaccine. This collaborative approach is expected to not only enhance the vaccine’s effectiveness but also streamline the regulatory approval process, ensuring that the vaccine reaches the public as quickly as possible.
Overall, while challenges remain in the development timeline, Kaiser’s dedication to research and comm
Expected Timeline for Release
The development of the RSV (Respiratory Syncytial Virus) vaccine by Kaiser Permanente is highly anticipated in the medical community. Given the ongoing research and the urgency due to increasing RSV infections, understanding the expected timeline for release is crucial.
Kaiser has been making significant strides in their RSV vaccine research. Although specific dates for the release are yet to be disclosed, experts predict that with the current momentum and support, we might see the vaccine enter the market as early as 2025. This timeline is contingent on various factors such as ongoing clinical trials, regulatory approvals, and manufacturing capabilities.
Moreover, the potential approval of the vaccine will depend on the results of the Phase III clinical trials, which are critical for safety and efficacy validation. This could mean a waiting period of several months after the trials conclude. As the situation stands, stakeholders in public health are encouraged to remain optimistic yet cautious as further developments unfold.
Challenges in RSV Vaccine Development
Developing a vaccine for Respiratory Syncytial Virus (RSV) presents several significant challenges that researchers and pharmaceutical companies must overcome. One of the major hurdles is understanding the complex biology of RSV itself. The virus has multiple subtypes, making it difficult to create a vaccine that is effective against all variants.
Another issue is the target population for the vaccine. RSV primarily affects infants and elderly individuals, groups that often have different immune responses. Designing a vaccine that can elicit a strong enough response in these vulnerable populations is a daunting task, requiring innovative approaches to immunization.
Moreover, the development timeline for vaccines can be extensive, involving multiple phases of clinical trials to ensure safety and efficacy. These trials can be complicated by the need for precise diagnostic criteria and the inherent variability in disease severity among patients. As researchers navigate these complexities, collaborative efforts among scientists, health organizations, and reg
Impact of RSV Vaccine on Public Health
The development and distribution of the RSV vaccine will be a major advancement in public health, particularly for vulnerable populations such as infants, the elderly, and those with compromised immune systems. Respiratory Syncytial Virus (RSV) is known to cause severe respiratory illness in these groups, leading to hospitalizations and, in some cases, fatalities. By effectively vaccinating against RSV, we can significantly reduce the incidence of these serious health outcomes.
One of the most significant impacts of the RSV vaccine will likely be on healthcare systems. With fewer hospitalizations and outpatient visits related to RSV, healthcare providers can allocate resources more efficiently, ensuring better care for other medical conditions. This can also lead to substantial savings in healthcare costs associated with RSV management, making this vaccine not just a health priority but also an economic one.
Moreover, the broader community will benefit from increased herd immunity as more individuals become vaccinated. This will protect those who are unable to receive the vaccine due to medical reasons, creating a safer environment for everybody. Overall, the potential for the RSV vaccine to improve public health outcomes cannot be overstated, providing hope for a future with reduced respiratory illness burden.
Frequently Asked Questions
What is the RSV vaccine?
The RSV vaccine is designed to prevent respiratory syncytial virus (RSV), a common virus that can cause severe respiratory illness, especially in infants and elderly individuals.
Why is the RSV vaccine important?
The RSV vaccine is important because it can significantly reduce the incidence of severe RSV infections, leading to fewer hospitalizations and better health outcomes for vulnerable populations.
When is Kaiser expected to release the RSV vaccine?
While specific dates may vary based on regulatory approvals, Kaiser is expected to release information about the RSV vaccine in late 2023 or early 2024.
Who is the target audience for the RSV vaccine?
The target audience for the RSV vaccine primarily includes infants, young children, and elderly adults, who are at a higher risk for severe RSV infections.
How can people stay informed about the RSV vaccine from Kaiser?
People can stay informed by regularly checking Kaiser’s official website, subscribing to health newsletters, and consulting with their healthcare providers.
Are there any existing treatments for RSV?
Yes, while there is no specific antiviral treatment for RSV, supportive care is typically provided, including hydration and oxygen therapy for severe cases.
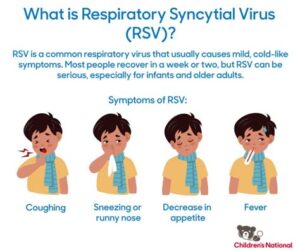
What are the symptoms of RSV infection?
Symptoms of RSV infection can include coughing, wheezing, difficulty breathing, fever, and congestion, which may vary in severity based on the individual’s health status.